Abstract
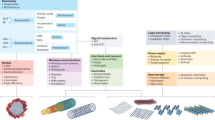
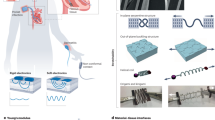
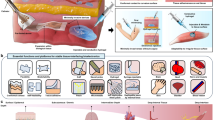
Advancements in bioelectronics are revolutionizing traditional healthcare by shifting the focus from in-hospital disease diagnosis and treatment to at-home continuous preventive care. This transformation integrates real-time health monitoring and point-of-care interventional therapies and enables artificial intelligence-based health management strategies. However, the mechanical mismatch between rigid bioelectronic devices and soft biological tissues presents important challenges, particularly in long-term applications, including poor adhesion, tissue degeneration, high noise level, signal interference and device instability. To address these challenges, soft bioelectronics — leveraging high-performance, tissue-mimicking and mechanically soft materials — has emerged as a disruptive solution. This Review highlights advancements in materials design and system-level integration strategies for soft bioelectronics, driving the development of next-generation digital healthcare technologies. We categorize materials design approaches, introduce fabrication techniques for soft bioelectronics and explore integration methods. Furthermore, we showcase applications of wearable and implantable soft bioelectronics, demonstrating their potential for continuous health monitoring and therapeutic interventions, ultimately enabling closed-loop health management.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Park, S., Garcia-Palacios, J., Cohen, A. & Varga, Z. From treatment to prevention: the evolution of digital healthcare. Nature 573, 7775 (2019).
Chen, C., Ding, S. & Wang, J. Digital health for aging populations. Nat. Med. 29, 1623–1630 (2023).
Chapman, R. & Middleton, J. The NHS long term plan and public health. BMJ 364, l218 (2019).
Sunwoo, S.-H., Ha, K.-H., Lee, S., Lu, N. & Kim, D.-H. Wearable and implantable soft bioelectronics: device designs and material strategies. Annu. Rev. Chem. Biomol. 12, 359–391 (2021).
Cho, K. W. et al. Soft bioelectronics based on nanomaterials. Chem. Rev. 122, 5068–5143 (2022).
Feiner, R. & Dvir, T. Tissue–electronics interfaces: from implantable devices to engineered tissues. Nat. Rev. Mater. 3, 17076 (2018).
Zhao, C., Park, J., Root, S. E. & Bao, Z. Skin-inspired soft bioelectronic materials, devices and systems. Nat. Rev. Bioeng. 2, 671–690 (2024).
Yuk, H., Wu, J. & Zhao, X. Hydrogel interfaces for merging humans and machines. Nat. Rev. Mater. 7, 935–952 (2022).
Kim, J. et al. Skin-interfaced wireless biosensors for perinatal and paediatric health. Nat. Rev. Bioeng. 1, 631–647 (2023).
Lin, M., Hu, H., Zhou, S. & Xu, S. Soft wearable devices for deep-tissue sensing. Nat. Rev. Mater. 7, 850–869 (2022).
Koo, J. H. et al. Electronic skin: opportunities and challenges in convergence with machine learning. Annu. Rev. Biomed. Eng. 26, 331–355 (2024).
Xu, C., Solomon, S. A. & Gao, W. Artificial intelligence-powered electronic skin. Nat. Mach. Intell. 5, 1344–1355 (2023).
Luo, Y. et al. Technology roadmap for flexible sensors. ACS Nano 17, 5211–5295 (2023).
Walter, J. R., Xu, S. & Rogers, J. A. From lab to life: how wearable devices can improve health equity. Nat. Commun. 15, 123 (2024).
Davis, N., Heikenfeld, J., Milla, C. & Javey, A. The challenges and promise of sweat sensing. Nat. Biotechnol. 42, 860–871 (2024).
Tang, X., Shen, H., Zhao, S., Li, N. & Liu, J. Flexible brain–computer interfaces. Nat. Electron. 6, 109–118 (2023).
Sunwoo, S.-H. et al. Soft bioelectronics for the management of cardiovascular diseases. Nat. Rev. Bioeng. 2, 8–24 (2024).
Dong, C. et al. Electrochemically actuated microelectrodes for minimally invasive peripheral nerve interfaces. Nat. Mater. 23, 969–976 (2024).
Jiang, H. et al. Finite deformation mechanics in buckled thin films on compliant supports. Proc. Natl Acad. Sci. USA 104, 15607–15612 (2007).
Sun, Y., Choi, W. M., Jiang, H., Huang, Y. Y. & Rogers, J. A. Controlled buckling of semiconductor nanoribbons for stretchable electronics. Nat. Nanotechnol. 1, 201–207 (2006).
Kim, D.-H. et al. Epidermal electronics. Science 333, 838–843 (2011).
Liu, J. et al. Syringe injectable electronics. Nat. Nanotechnol. 10, 629–636 (2015).
Wang, Z. et al. Kirigami-patterned highly stretchable conductors from flexible carbon nanotube-embedded polymer films. J. Mater. Chem. C 5, 8714–8722 (2017).
Blees, M. K. et al. Graphene kirigami. Nature 524, 204–207 (2015).
Matsuhisa, N., Chen, X., Bao, Z. & Someya, T. Materials and structural designs of stretchable conductors. Chem. Soc. Rev. 48, 2946–2966 (2019).
Han, M. et al. Three-dimensional piezoelectric polymer microsystems for vibrational energy harvesting, robotic interfaces and biomedical implants. Nat. Electron. 2, 26–35 (2019).
Zhang, L. et al. 3D morphable systems via deterministic microfolding for vibrational sensing, robotic implants, and reconfigurable telecommunication. Sci. Adv. 8, eade0838 (2022).
Zhang, L. et al. Skin-inspired, sensory robots for electronic implants. Nat. Commun. 15, 4777 (2024).
Kim, Y. et al. Chip-less wireless electronic skins by remote epitaxial freestanding compound semiconductors. Science 377, 859–864 (2022).
Liu, Z. et al. A three-dimensionally architected electronic skin mimicking human mechanosensation. Science 384, 987–994 (2024).
Lacour, S. P., Wagner, S., Huang, Z. & Suo, Z. Stretchable gold conductors on elastomeric substrates. Appl. Phys. Lett. 82, 2404–2406 (2003).
Minev, I. R. et al. Electronic dura mater for long-term multimodal neural interfaces. Science 347, 159–163 (2015).
Park, J. et al. Electromechanical cardioplasty using a wrapped elasto-conductive epicardial mesh. Sci. Transl. Med. 8, 344ra86 (2016).
Choi, S. et al. Highly conductive, stretchable and biocompatible Ag–Au core–sheath nanowire composite for wearable and implantable bioelectronics. Nat. Nanotechnol. 13, 1048–1056 (2018).
Jung, D. et al. Highly conductive and elastic nanomembrane for skin electronics. Science 373, 1022–1026 (2021).
Siddiqui, S. et al. A durable and stable piezoelectric nanogenerator with nanocomposite nanofibers embedded in an elastomer under high loading for a self-powered sensor system. Nano Energy 30, 434–442 (2016).
Lin, Z., Li, T., Yang, S., Ji, B. & Wang, Z. Revolutionizing flexible electronics with liquid metal innovations. Device 2, 100331 (2024).
Lee, W. et al. Universal assembly of liquid metal particles in polymers enables elastic printed circuit board. Science 378, 637–641 (2022).
Zhou, Y. et al. Giant magnetoelastic effect in soft systems for bioelectronics. Nat. Mater. 20, 1670–1676 (2021).
Kim, S. H. et al. Strain-invariant stretchable radio-frequency electronics. Nature 629, 1047–1054 (2024).
Kim, D. C. et al. Intrinsically stretchable quantum dot light-emitting diodes. Nat. Electron. 7, 365–374 (2024).
Xu, J. et al. Highly stretchable polymer semiconductor films through the nanoconfinement effect. Science 355, 59–64 (2017).
Shim, H. et al. Elastic integrated electronics based on a stretchable n-type elastomer–semiconductor–elastomer stack. Nat. Electron. 6, 349–359 (2023).
Tan, P. et al. Solution-processable, soft, self-adhesive, and conductive polymer composites for soft electronics. Nat. Commun. 13, 358 (2022).
Han, W. B. et al. Ultra-stretchable and biodegradable elastomers for soft, transient electronics. Nat. Commun. 14, 2263 (2023).
Ohm, Y. et al. An electrically conductive silver–polyacrylamide–alginate hydrogel composite for soft electronics. Nat. Electron. 4, 185–192 (2021).
Lu, Y. et al. Stretchable graphene–hydrogel interfaces for wearable and implantable bioelectronics. Nat. Electron. 7, 51–65 (2024).
Jin, S. et al. Injectable tissue prosthesis for instantaneous closed-loop rehabilitation. Nature 623, 58–65 (2023).
Jiang, Y. et al. Topological supramolecular network enabled high-conductivity, stretchable organic bioelectronics. Science 375, 1411–1417 (2022).
Zhou, T. et al. 3D printable high-performance conducting polymer hydrogel for all-hydrogel bioelectronic interfaces. Nat. Mater. 22, 895–902 (2023).
Li, P. et al. N-type semiconducting hydrogel. Science 384, 557–563 (2024).
Peng, H., Xin, Y., Xu, J., Liu, H. & Zhang, J. Ultra-stretchable hydrogels with reactive liquid metals as asymmetric force-sensors. Mater. Horiz. 6, 618–625 (2019).
Jing, X., Mi, H.-Y., Peng, X.-F. & Turng, L.-S. Biocompatible, self-healing, highly stretchable polyacrylic acid/reduced graphene oxide nanocomposite hydrogel sensors via mussel-inspired chemistry. Carbon 136, 63–72 (2018).
Qin, Z. et al. Carbon nanotubes/hydrophobically associated hydrogels as ultrastretchable, highly sensitive, stable strain, and pressure sensors. ACS Appl. Mater. Interfaces 12, 4944–4953 (2020).
Deng, J. et al. Electrical bioadhesive interface for bioelectronics. Nat. Mater. 20, 229–236 (2021).
Yang, J., Zhao, Z., Wang, S., Guo, Y. & Liu, Y. Insight into high-performance conjugated polymers for organic field-effect transistors. Chem 4, 2748–2785 (2018).
Van der Zee, B., Li, Y., Wetzelaer, G.-J. A. H. & Blom, P. W. M. Efficiency of polymer light-emitting diodes: a perspective. Adv. Mater. 34, 2108887 (2022).
Li, G., Zhu, R. & Yang, Y. Polymer solar cells. Nat. Photon. 6, 153–161 (2012).
Fan, X. et al. PEDOT:PSS for flexible and stretchable electronics: modifications, strategies, and applications. Adv. Sci. 6, 1900813 (2019).
Baker, C. O., Huang, X., Nelson, W. & Kaner, R. B. Polyaniline nanofibers: broadening applications for conducting polymers. Chem. Soc. Rev. 5, 1510–1525 (2017).
Liu, Y. & Wu, F. Synthesis and application of polypyrrole nanofibers: a review. Nanoscale Adv. 5, 3606–3618 (2023).
Boyle, C. J. et al. Tuning charge transport dynamics via clustering of doping in organic semiconductor thin films. Nat. Commun. 10, 2827 (2019).
Li, Y., Sonar, P., Murphy, L. & Hong, W. High mobility diketopyrrolopyrrole (DPP)-based organic semiconductor materials for organic thin film transistors and photovoltaics. Energy Environ. Sci. 6, 1684–1710 (2013).
Costa, C. M. et al. Smart and multifunctional materials based on electroactive poly(vinylidene fluoride): recent advances and opportunities in sensors, actuators, energy, environmental, and biomedical applications. Chem. Rev. 123, 11392–11487 (2023).
Liu, X.-J., Zheng, M.-S., Chen, G., Dang, Z.-M. & Zha, J.-W. High-temperature polyimide dielectric materials for energy storage: theory, design, preparation and properties. Energy Environ. Sci. 15, 56–81 (2022).
Zheng, Y., Zhang, S., Tok, J. B.-H. & Bao, Z. Molecular design of stretchable polymer semiconductors: current progress and future directions. J. Am. Chem. Soc. 144, 4699–4715 (2022).
Lipomi, D. J. Stretchable figures of merit in deformable electronics. Adv. Mater. 28, 4180–4183 (2016).
Zhuo, Z. et al. Intrinsically stretchable fully π-conjugated polymer film via fluid conjugated molecular external-plasticizing for flexible light-emitting diodes. Nat. Commun. 15, 7990 (2024).
Kim, J.-H. & Park, J.-W. Intrinsically stretchable organic light-emitting diodes. Sci. Adv. 7, eabd9715 (2021).
Hosseini, E., Kollath, V. O. & Karan, K. The key mechanism of conductivity in PEDOT:PSS thin films exposed by anomalous conduction behaviour upon solvent-doping and sulfuric acid post-treatment. J. Mater. Chem. C 8, 3982–3990 (2020).
Wang, Y. et al. A highly stretchable, transparent, and conductive polymer. Sci. Adv. 3, e1602076 (2017).
Lingstedt, L. V. et al. Effect of DMSO solvent treatments on the performance of PEDOT:PSS based organic electrochemical transistors. Adv. Electron. Mater. 5, 1800804 (2019).
Chen, R. et al. PEDOT:PSS as stretchable conductors with good wettability on the substrate through the simultaneous plasticization and secondary doping with a cationic or anionic surfactant. Macromolecules 12, 4967–4978 (2022).
Mun, J. et al. Conjugated carbon cyclic nanorings as additives for intrinsically stretchable semiconducting polymers. Adv. Mater. 31, 1903912 (2019).
Yu, Z., Xia, Y., Du, D. & Ouyang, J. PEDOT:PSS films with metallic conductivity through a treatment with common organic solutions of organic salts and their application as a transparent electrode of polymer solar cells. ACS Appl. Mater. Interfaces 8, 11629–11638 (2016).
Li, W., Li, Y., Song, Z., Wang, Y.-X. & Hu, W. PEDOT-based stretchable optoelectronic materials and devices for bioelectronic interfaces. Chem. Soc. Rev. 53, 10575–10603 (2024).
Liu, Y. et al. Soft and elastic hydrogel-based microelectronics for localized low-voltage neuromodulation. Nat. Biomed. Eng. 3, 58–68 (2019).
Lu, B. et al. Pure PEDOT:PSS hydrogels. Nat. Commun. 10, 1043 (2019).
Feing, V. R., Tran, H., Lee, M. & Bao, Z. Mechanically tunable conductive interpenetrating network hydrogels that mimic the elastic moduli of biological tissue. Nat. Commun. 9, 2740 (2018).
Oh, J. Y. et al. Intrinsically stretchable and healable semiconducting polymer for organic transistors. Nature 539, 411–415 (2016).
Zheng, Y. et al. An intrinsically stretchable high-performance polymer semiconductor with low crystallinity. Adv. Funct. Mater. 29, 1905340 (2019).
Liu, D. et al. Incorporating conjugated rigid fused-rings with bulky side groups. J. Am. Chem. Soc. 143, 11679–11689 (2021).
Liu, W. et al. High-efficiency stretchable light-emitting polymers from thermally activated delayed fluorescence. Nat. Mater. 22, 737–745 (2023).
Mun, J. et al. Effect of nonconjugated spacers on mechanical properties of semiconducting polymers for stretchable transistors. Adv. Funct. Mater. 28, 1804222 (2018).
Zhang, S. et al. Molecular origin of strain-induced chain alignment in PDPP-based semiconducting polymeric thin films. Adv. Funct. Mater. 31, 2100161 (2021).
Chen, W. et al. Elastic–plastic fully π-conjugated polymer with excellent energy dissipation capacity for ultra-deep-blue flexible polymer light-emitting diodes with CIEy = 0.04. Adv. Mater. 36, 2402708 (2024).
Yue, H. et al. In situ continuous hydrogen-bonded engineering for intrinsically stretchable and healable high-mobility polymer semiconductors. Sci. Adv. 10, eadq0171 (2024).
Mun, J. et al. A design strategy for high mobility stretchable polymer semiconductors. Nat. Commun. 12, 3572 (2021).
Yu, X. et al. Intrinsically stretchable polymer semiconductors with good ductility and high charge mobility through reducing the central symmetry of the conjugated backbone units. Adv. Mater. 35, 2209896 (2023).
Chen, J. et al. Molecular design of multifunctional integrated polymer semiconductors with intrinsic stretchability, high mobility, and intense luminescence. Adv. Mater. 36, 2305987 (2024).
Xue, X. et al. Conjugated polymer-based photo-crosslinker for efficient photo-patterning of polymer semiconductors. Adv. Mater. 36, 2407305 (2024).
Zheng, Y. et al. A molecular design approach towards elastic and multifunctional polymer electronics. Nat. Commun. 12, 5701 (2021).
Choi, S., Han, S. I., Kim, D., Hyeon, T. & Kim, D.-H. High-performance stretchable conductive nanocomposites: materials, processes, and device applications. Chem. Soc. Rev. 48, 1566–1595 (2019).
Park, M., Park, J. & Jeong, U. Design of conductive composite elastomers for stretchable electronics. Nano Today 9, 244–260 (2014).
Kim, N. et al. Elastic conducting polymer composites in thermoelectric modules. Nat. Commun. 11, 1424 (2020).
Murray, C. B., Norris, D. J. & Bawendi, M. G. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J. Am. Chem. Soc. 115, 8706–8715 (1993).
Park, J. et al. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 3, 891–895 (2004).
Kim, Y. et al. Stretchable nanoparticle conductors with self-organized conductive pathways. Nature 500, 59–63 (2013).
José Andrés, L. et al. Rapid synthesis of ultra-long silver nanowires for tailor-made transparent conductive electrodes: proof of concept in organic solar cells. Nanotechnology 26, 265201 (2015).
Moon, G. D. et al. Highly stretchable patterned gold electrodes made of Au nanosheets. Adv. Mater. 25, 1707–2712 (2013).
Lim, C. et al. Facile and scalable synthesis of whiskered gold nanosheets for stretchable, conductive, and biocompatible nanocomposites. ACS Nano 16, 10431–10442 (2022).
Liu, K. et al. Low-voltage intrinsically stretchable organic transistor amplifiers for ultrasensitive electrophysiological signal detection. Adv. Mater. 35, 2207006 (2023).
Tropp, J. et al. Conducting polymer nanoparticles with intrinsic aqueous dispersibility for conductive hydrogels. Adv. Mater. 36, 2306691 (2024).
Sunwoo, S.-H. et al. Stretchable low-impedance conductor with Ag–Au–Pt core–shell–shell nanowires and in situ formed Pt nanoparticles for wearable and implantable device. ACS Nano 17, 7550–7561 (2023).
Liu, C.-H. & Yu, X. Silver nanowire-based transparent, flexible, and conductive thin film. Nanoscale Res. Lett. 6, 75 (2011).
Jiang, Z. et al. Highly stretchable metallic nanowire networks reinforced by the underlying randomly distributed elastic polymer nanofibers via interfacial adhesion improvement. Adv. Mater. 31, 1903446 (2019).
Jung, D. et al. Metal-like stretchable nanocomposite using locally-bundled nanowires for skin-mountable devices. Adv. Mater. 35, 2303458 (2023).
Song, S. et al. Photothermal lithography for realizing a stretchable multilayer electronic circuit using a laser. ACS Nano 17, 21443–21454 (2023).
Zhao, Y. et al. Soft strain-insensitive bioelectronics featuring brittle materials. Science 378, 1222–1227 (2022).
Lim, C. et al. Highly conductive and stretchable hydrogel nanocomposite using whiskered gold nanosheets for soft bioelectronics. Adv. Mater. 36, 2407931 (2024).
Zhao, Y. et al. A self-healing electrically conductive organogel composite. Nat. Electron. 6, 206–215 (2023).
Yuk, H., Lu, B. & Zhao, X. Hydrogel bioelectronics. Chem. Soc. Rev. 48, 1642–1667 (2019).
Wang, Z., Volinsky, A. A. & Gallant, N. D. Crosslinking effect on polydimethylsiloxane elastic modulus measured by custom-built compression instrument. J. Appl. Polym. Sci. 131, 41050 (2014).
Harito, C., Bavykin, D. V., Yuliarto, B., Dipojono, H. K. & Walsh, F. C. Polymer nanocomposites having a high filler content: synthesis, structures, properties, and applications. Nanoscale 11, 4653–4682 (2019).
Li, G. et al. Highly conducting and stretchable double-network hydrogel for soft bioelectronics. Adv. Mater. 34, 2200261 (2022).
Yuk, H. et al. Dry double-sided tape for adhesion of wet tissues and devices. Nature 575, 169–174 (2019).
Zhang, W. et al. Catechol-functionalized hydrogels: biomimetic design, adhesion mechanism, and biomedical applications. Chem. Soc. Rev. 49, 433–464 (2020).
Vo, N. T. P. et al. Autonomous self-healing supramolecular polymer transistors for skin electronics. Nat. Commun. 15, 3433 (2024).
Bae, J.-Y. et al. A biodegradable and self-deployable electronic tent electrode for brain cortex interfacing. Nat. Electron. 7, 815–828 (2024).
Xu, Y. et al. Phase-separated porous nanocomposite with ultralow percolation threshold for wireless bioelectronics. Nat. Nanotechnol. 19, 1158–1167 (2024).
Matsuhisa, N. et al. Printable elastic conductors by in situ formation of silver nanoparticles from silver flakes. Nat. Mater. 16, 834–840 (2017).
Jiang, Z. et al. A 1.3-micrometre-thick elastic conductor for seamless on-skin and implantable sensors. Nat. Electron. 5, 784–793 (2022).
Rosset, S., Niklaus, M., Dubois, P. & Shea, H. R. Metal ion implantation for the fabrication of stretchable electrodes on elastomers. Adv. Funct. Mater. 19, 470–478 (2009).
Akter, T. & Kim, W. S. Reversibly stretchable transparent conductive coatings of spray-deposited silver nanowires. ACS Appl. Mater. Interfaces 4, 1855–1859 (2012).
Xu, F. & Zhu, Y. Highly conductive and stretchable silver nanowire conductors. Adv. Mater. 24, 5117–5122 (2012).
Liu, H.-S., Pan, B.-C. & Liou, G.-S. Highly transparent AgNW/PDMS stretchable electrodes for elastomeric electrochromic devices. Nanoscale 9, 2633–2639 (2017).
Yu, Z. et al. Highly flexible silver nanowire electrodes for shape-memory polymer light-emitting diodes. Adv. Mater. 23, 664–668 (2011).
Koo, J. H. et al. A vacuum-deposited polymer dielectric for wafer-scale stretchable electronics. Nat. Electron. 6, 137–145 (2023).
Xu, J. et al. Multi-scale ordering in highly stretchable polymer semiconducting films. Nat. Mater. 18, 594–601 (2019).
Wang, W. et al. Neuromorphic sensorimotor loop embodied by monolithically integrated low voltage, soft e-skin. Science 380, 735–742 (2023).
Wang, W. et al. Strain-insensitive intrinsically stretchable transistors and circuits. Nat. Electron. 4, 143–150 (2021).
Guan, Y.-S. et al. Elastic electronics based on micromesh-structured rubbery semiconductor films. Nat. Electron. 5, 881–892 (2022).
Zhong, D. et al. High-speed and large-scale intrinsically stretchable integrated circuits. Nature 627, 313–320 (2024).
Gong, S. et al. A wearable and highly sensitive pressure sensor with ultrathin gold nanowires. Nat. Commun. 5, 3132 (2014).
Cheng, Y., Wang, S., Wang, R., Sun, J. & Gao, L. Copper nanowire based transparent conductive films with high stability and superior stretchability. J. Mater. Chem. C 2, 5309–5316 (2014).
Shin, Y. et al. Low-impedance tissue–device interface using homogeneously conductive hydrogels chemically bonded to stretchable bioelectronics. Sci. Adv. 10, eadi7724 (2024).
Li, M., Li, H., Zhong, W., Zhao, Q. & Wang, D. Stretchable conductive polypyrrole/polyurethane (PPy/PU) strain sensor with netlike microcracks for human breath detection. ACS Appl. Mater. Interfaces 6, 1313–1319 (2014).
Liu, Z., Qian, Z., Song, J. & Zhang, Y. Conducting and stretchable composites using sandwiched graphene–carbon nanotube hybrids and styrene–butadiene rubber. Carbon 149, 181–189 (2019).
Jung, D. et al. Adaptive self-organization of nanomaterials enables strain-insensitive resistance of stretchable metallic nanocomposites. Adv. Mater. 34, 2200980 (2022).
Seo, H. et al. Durable and fatigue-resistant soft peripheral neuroprosthetics for in vivo bi-directional signaling. Adv. Mater. 33, 2007346 (2021).
Ma, Z. et al. Permeable superelastic liquid–metal fibre mat enables biocompatible and monolithic stretchable electronics. Nat. Mater. 20, 859–868 (2021).
Deng, Y. et al. Stretchable liquid metal based biomedical devices. npj Flex. Electron. 8, 12 (2024).
Xu, Y. et al. Porous liquid metal elastomer composites with high leakage resistance and antimicrobial property for skin-interfaced bioelectronics. Sci. Adv. 9, eadf0575 (2023).
Ho, D. H., Hu, C., Li, L. & Bartlett, M. D. Soft electronic vias and interconnects through rapid three-dimensional assembly of liquid metal microdroplets. Nat. Electron. 7, 1015–1024 (2024).
Paulsen, B. D., Tybrandt, K., Stavrinidou, E. & Rivnay, J. Organic mixed ionic–electronic conductors. Nat. Mater. 19, 13–26 (2020).
Chong, J. et al. Highly conductive tissue-like hydrogel interface through template-directed assembly. Nat. Commun. 14, 2206 (2023).
Sim, K. et al. Fully rubbery integrated electronics from high effective mobility intrinsically stretchable semiconductors. Sci. Adv. 5, eaav5749 (2019).
Yang, D. et al. High-performance carbon nanotube field-effect transistors with electron mobility of 39.4 cm2 V−1 s−1 using anion–π interaction doping. Carbon 203, 761–769 (2023).
Liu, N. et al. Ultratransparent and stretchable graphene electrodes. Sci. Adv. 3, e1700159 (2017).
Liang, J. et al. Intrinsically stretchable and transparent thin-film transistors based on printable silver nanowires, carbon nanotubes and an elastomeric dielectric. Nat. Commun. 6, 7647 (2015).
Wang, B. et al. High-k gate dielectrics for emerging flexible and stretchable electronics. Chem. Rev. 118, 5690–5754 (2018).
Yoo, H. et al. Janus CoMOF-SEBS membrane for bifunctional dielectric layer in triboelectric nanogenerators. Adv. Sci. 11, 2307656 (2024).
Liu, G. et al. Enhanced dielectric performance of PDMS-based three-phase percolative nanocomposite films incorporating a high dielectric constant ceramic and conductive multi-walled carbon nanotubes. J. Mater. Chem. C 6, 10829–10837 (2018).
Liu, S. et al. Research progress on dielectric properties of PU and its application on capacitive sensors and OTFTs. React. Funct. Polym. 181, 105420 (2022).
Matsuno, R. et al. Relationship between the relative dielectric constant and the monomer sequence of acrylonitrile in rubber. ACS Omega 5, 16255–16262 (2020).
Kim, J. Y. Phase behavior of binary and ternary fluoropolymer (PVDF-HFP) solutions for single-ion conductors. RSC Adv. 12, 21160–21171 (2022).
Ankit et al. High-k, ultrastretchable self-enclosed ionic liquid–elastomer composites for soft robotics and flexible electronics. ACS Appl. Mater. Interfaces 12, 37561–37570 (2020).
Yang, D. et al. Improved electromechanical properties of NBR dielectric composites by poly(dopamine) and silane surface functionalized TiO2 nanoparticles. J. Mater. Chem. C 4, 7724–7734 (2016).
Kang, J. et al. Ultrathin, solvent-resistant dielectric for monolithic fabrication of low-power, intrinsically stretchable active-matrix electronic skin. Device 2, 100426 (2024).
Chang, S. et al. Flexible and stretchable light-emitting diodes and photodetectors for human-centric optoelectronics. Chem. Rev. 124, 768–859 (2024).
Dai, Y., Hu, H., Wang, M., Xu, J. & Wang, S. Stretchable transistors and functional circuits for human-integrated electronics. Nat. Electron. 4, 17–29 (2021).
Kim, H. J., Choi, H., Kim, D.-H. & Son, D. Stretchable functional nanocomposites for soft implantable bioelectronics. Nano Lett. 24, 8453–8464 (2024).
Bian, Y. et al. Patterning techniques based on metallized electrospun nanofibers for advanced stretchable electronics. Adv. Sci. 11, 2309735 (2024).
Ma, J. et al. Shaping a soft future: patterning liquid metals. Adv. Mater. 35, 2205196 (2023).
Wu, H. et al. Fabrication techniques for curved electronics on arbitrary surfaces. Adv. Mater. Technol. 5, 2000093 (2020).
Won, D. et al. Digital selective transformation and patterning of highly conductive hydrogel bioelectronics by laser-induced phase separation. Sci. Adv. 8, eabo3209 (2022).
Zhang, X.-R. Recent progress of patterned electrodes in wearable electronics: fabrication and application. J. Phys. D Appl. Phys. 57, 013001 (2023).
Zhou, W. et al. Soft and stretchable organic bioelectronics for continuous intraoperative neurophysiological monitoring during microsurgery. Nat. Biomed. Eng. 7, 1270–1281 (2023).
Zhuang, Q. et al. Wafer-patterned, permeable, and stretchable liquid metal microelectrodes for implantable bioelectronics with chronic biocompatibility. Sci. Adv. 9, eadg8602 (2023).
Lee, G.-H. et al. Conductance stable and mechanically durable bi-layer EGaIn composite-coated stretchable fiber for 1D bioelectronics. Nat. Commun. 14, 4173 (2023).
Yan, Z. et al. Highly stretchable van der Waals thin films for adaptable and breathable electronic membranes. Science 375, 852–859 (2022).
Sim, K. et al. An epicardial bioelectronic patch made from soft rubbery materials and capable of spatiotemporal mapping of electrophysiological activity. Nat. Electron. 3, 775–784 (2020).
Choudhary, K. et al. Comparison of the mechanical properties of a conjugated polymer deposited using spin coating, interfacial spreading, solution shearing, and spray coating. ACS Appl. Mater. Interfaces 13, 51436–51446 (2021).
Soe, H. M., Manaf, A. A., Matsuda, A. & Jaafar, M. Performance of a silver nanoparticles-based polydimethylsiloxane composite strain sensor produced using different fabrication methods. Sens. Actuat. A Phys. 329, 112793 (2021).
Dey, R. et al. Graphene-based electrodes for ECG signal monitoring: fabrication methodologies, challenges and future directions. Cogent Eng. 10, 2246750 (2023).
Xu, Z. et al. A highly-adhesive and self-healing elastomer for bio-interfacial electrode. Adv. Funct. Mater. 31, 2006432 (2021).
Liu, S. et al. Self-healing, robust, and stretchable electrode by direct printing on dynamic polyurea surface at slightly elevated temperature. Adv. Funct. Mater. 31, 2102225 (2021).
Lee, G.-H. et al. Large-area photo-patterning of initially conductive EGaIn particle-assembled film for soft electronics. Mater. Today 67, 84–94 (2023).
Lee, T. et al. Large-area synthesis of ultrathin, flexible, and transparent conductive metal-organic framework thin films via a microfluidic-based solution shearing process. Adv. Mater. 34, 2107696 (2022).
Du, X. et al. A review of inkjet printing technology for personalized-healthcare wearable devices. J. Mater. Chem. C 10, 14091–14115 (2022).
Liu, Y. et al. Recent advances in inkjet-printing technologies for flexible/wearable electronics. Nanoscale 15, 6025–6051 (2023).
Zou, Z. et al. 3D printing of liquid metals: recent advancements and challenges. Adv. Funct. Mater. 33, 2213312 (2023).
Ge, G., Wang, Q., Zhang, Y.-Z., Alshareef, H. N. & Dong, X. 3D printing of hydrogels for stretchable ionotronic devices. Adv. Funct. Mater. 31, 2107437 (2021).
Gao, Z. et al. Advances in wearable strain sensors based on electrospun fibers. Adv. Funct. Mater. 33, 2214265 (2023).
Wang, Y., Yokota, T. & Someya, T. Electrospun nanofiber-based soft electronics. NPG Asia Mater. 13, 22 (2021).
Oh, B. et al. 3D printable and biocompatible PEDOT:PSS-ionic liquid colloids with high conductivity for rapid on-demand fabrication of 3D bioelectronics. Nat. Commun. 15, 5839 (2024).
Neumann, T. V. & Dickey, M. D. Liquid metal direct write and 3D printing: a review. Adv. Mater. Technol. 5, 2000070 (2020).
Saadi, M. A. S. R. et al. Direct ink writing: a 3D printing technology for diverse materials. Adv. Mater. 34, 2108855 (2022).
Park, J., Lee, Y., Lee, H. & Ko, H. Transfer printing of electronic functions on arbitrary complex surfaces. ACS Nano 14, 12–20 (2020).
Huang, Z. & Lin, Y. Transfer printing technologies for soft electronics. Nanoscale 14, 16749–16760 (2022).
Sakorikar, T. et al. A guide to printed stretchable conductors. Chem. Rev. 124, 860–888 (2024).
Havenko, S., Czubak, J., Piskozub, Y., Uhryn, Y. & Labetska, M. Modeling the process of ink transfer from the gravure printing plate to the printing substrate. J. Print Media Technol. Res. 13, 97–105 (2024).
Liedert, C. et al. Roll-to-roll manufacturing of integrated immunodetection sensors. ACS Sens. 5, 2010–2017 (2020).
Veerapandian, S. et al. Printable inks and deformable electronic array devices. Nanoscale Horiz. 7, 663–681 (2022).
Kim, D. W., Kong, M. & Jeong, U. Interface design for stretchable electronic devices. Adv. Sci. 8, 2004170 (2021).
Ahn, J. et al. Illuminating recent progress in nanotransfer printing: core principles, emerging applications, and future perspectives. Adv. Sci. 11, 2303704 (2024).
Bellani, S. et al. Solution-processed two-dimensional materials for next-generation photovoltaics. Chem. Soc. Rev. 50, 11870–11965 (2021).
Sun, L. et al. All-solution-processed ultraflexible wearable sensor enabled with universal trilayer structure for organic optoelectronic devices. Sci. Adv. 10, eadk9460 (2024).
Huang, J. et al. Intrinsically stretchable, semi-transparent organic photovoltaics with high efficiency and mechanical robustness via a full-solution process. Energy Environ. Sci. 16, 1251–1263 (2023).
Oh, J. Y. et al. Stretchable self-healable semiconducting polymer film for active-matrix strain-sensing array. Sci. Adv. 5, eaav3097 (2019).
Yang, D. et al. Double-microcrack coupling stretchable neural electrode for electrophysiological communication. Adv. Funct. Mater. 33, 2300412 (2023).
Lee, Y. et al. Standalone real-time health monitoring patch based on a stretchable organic optoelectronic system. Sci. Adv. 7, eabg9180 (2021).
Zhu, J. et al. Tuning strain sensor performance via programmed thin-film crack evolution. ACS Appl. Mater. Interfaces 13, 38105–38113 (2021).
Sun, Y.-C., Boero, G. & Brugger, J. Stretchable conductors fabricated by stencil lithography and centrifugal force-assisted patterning of liquid metal. ACS Appl. Electron. Mater. 3, 5423–5432 (2021).
Liu, S., Shah, D. S. & Kramer-Bottiglio, R. Highly stretchable multilayer electronic circuits using biphasic gallium–indium. Nat. Mater. 20, 851–858 (2021).
Kim, M. et al. Nanowire-assisted freestanding liquid metal thin-film patterns for highly stretchable electrodes on 3D surfaces. npj Flex. Electron. 6, 99 (2022).
Xu, C., Ma, B., Yuan, S., Zhao, C. & Liu, H. High-resolution patterning of liquid metal on hydrogel for flexible, stretchable, and self-healing electronics. Adv. Electron. Mater. 6, 1900721 (2020).
Kim, D. W. et al. Fabrication of practical deformable displays: advances and challenges. Light Sci. Appl. 12, 61 (2023).
Wang, S. et al. Skin electronics from scalable fabrication of an intrinsically stretchable transistor array. Nature 555, 83–88 (2018).
Li, J. et al. Vertically stacked skin-like active-matrix display with ultrahigh aperture ratio. Light Sci. Appl. 13, 177 (2024).
Chung, H. U. et al. Skin-interfaced biosensors for advanced wireless physiological monitoring in neonatal and pediatric intensive-care units. Nat. Med. 26, 418–429 (2020).
Zhou, S. et al. Transcranial volumetric imaging using a conformal ultrasound patch. Nature 629, 810–830 (2024).
Kwon, K. et al. An on-skin platform for wireless monitoring of flow rate, cumulative loss and temperature of sweat in real time. Nat. Electron. 4, 302–312 (2021).
Tu, J. et al. A wireless patch for the monitoring of C-reactive protein in sweat. Nat. Biomed. Eng. 7, 1293–1306 (2023).
Kim, M., Park, J. J., Cho, C. & Ko, S. H. Liquid metal based stretchable room temperature soldering sticker patch for stretchable electronics integration. Adv. Funct. Mater. 33, 2303286 (2023).
Tang, L., Yang, S., Zhang, K. & Jiang, X. Skin electronics from biocompatible in situ welding enabled by intrinsically sticky conductors. Adv. Sci. 9, 2202043 (2022).
Oh, J.-Y. et al. Skin electronics from biocompatible in situ welding enabled by intrinsically sticky conductors. ACS Appl. Mater. Interfaces 14, 49303–49312 (2022).
Zheng, S. et al. Pressure-stamped stretchable electronics using a nanofibre membrane containing semi-embedded liquid metal particles. Nat. Electron. 7, 576–585 (2024).
Lopes, P. A., Santos, B. C., de Almeida, A. T. & Tavakoli, M. Reversible polymer–gel transition for ultra-stretchable chip-integrated circuits through self-soldering and self-coating and self-healing. Nat. Commun. 12, 4666 (2021).
Jiang, Y. et al. A universal interface for plug-and-play assembly of stretchable devices. Nature 614, 456–462 (2023).
Oh, S. et al. Softening implantable bioelectronics: material designs, applications, and future directions. Biosens. Bioelectron. 258, 116328 (2024).
Lee, W. et al. Nonthrombogenic, stretchable, active multielectrode array for electroanatomical mapping. Sci. Adv. 4, eaau2426 (2018).
Oh, H. et al. High density integration of stretchable inorganic thin film transistors with excellent performance and reliability. Nat. Commun. 13, 4963 (2022).
Zhuang, Q. et al. Permeable, three-dimensional integrated electronic skins with stretchable hybrid liquid metal solders. Nat. Electron. 7, 598–609 (2024).
Min, H. et al. Highly air/water-permeable hierarchical mesh architectures for stretchable underwater electronic skin patches. ACS Appl. Mater. Interfaces 12, 14425–14432 (2020).
Yeon, H. et al. Long-term reliable physical health monitoring by sweat pore-inspired perforated electronic skins. Sci. Adv. 7, eabg8459 (2021).
Dominguez-Alfaro, A. et al. Light-based 3D multi-material printing of micro-structured bio-shaped, conducting and dry adhesive electrodes for bioelectronics. Adv. Sci. 11, 2306424 (2024).
Lee, S. et al. A shape-morphing cortex-adhesive sensor for closed-loop transcranial ultrasound neurostimulation. Nat. Electron. 7, 800–814 (2024).
Baik, S. et al. A wet-tolerant adhesive patch inspired by protuberances in suction cups of octopi. Nature 546, 396–404 (2017).
Wu, J. et al. Adhesive anti-fibrotic interfaces on diverse organs. Nature 630, 360–367 (2024).
Jeong, J. et al. Materials and optimized designs for human–machine interfaces via epidermal electronics. Adv. Mater. 25, 6839–6846 (2013).
Li, Y. et al. Achieving tissue-level softness on stretchable electronics through a generalizable soft interlayer design. Nat. Commun. 14, 4488 (2023).
Song, S. et al. Deployment of an electrocorticography system with a soft robotic actuator. Sci. Robot. 8, eadd1002 (2023).
Nam, S. et al. Needle-like multifunctional biphasic microfiber for minimally invasive implantable bioelectronics. Adv. Mater. 36, 2404101 (2024).
Hu, S., Wang, L., Liu, S. & Yin, L. Recent development of implantable chemical sensors utilizing flexible and biodegradable materials for biomedical applications. ACS Nano 18, 3969–3995 (2024).
Zhang, P., Zhu, B., Du, P. & Travas-Sejdic, J. Electrochemical and electrical biosensors for wearable and implantable electronics based on conducting polymers and carbon-based materials. Chem. Rev. 124, 722–767 (2024).
de Marzo, G. et al. Sustainable electronic biomaterials for body-compliant devices: challenges and perspectives for wearable bio-mechanical sensors and body energy harvesters. Nano Energy 123, 109336 (2024).
Cha, G. D., Kim, D.-H. & Kim, D. C. Wearable and implantable light-emitting diodes and their biomedical applications. Korean J. Chem. Eng. 41, 1–24 (2024).
Yang, Z., Song, H. & Ding, H. Advancements in implantable temperature sensors: materials, mechanisms, and biological applications. J. Semicond. 46, 011609 (2025).
Kar, A. et al. Wearable and implantable devices for drug delivery: applications and challenges. Biomaterials 283, 121435 (2022).
Zhang, Y. et al. Advances in wearable and implantable devices for wireless electrical stimulation therapy. Discov. Electron. 2, 6 (2025).
Lee, J. H., Lee, S., Kim, D. & Lee, K. J. Implantable micro-light-emitting diode (µLED)-based optogenetic interfaces toward human applications. Adv. Drug Deliv. Rev. 187, 114399 (2022).
Mazzotta, A., Carlotti, M. & Mattoli, V. Conformable on-skin devices for thermo-electro-tactile stimulation: materials, design, and fabrication. Mater. Adv. 2, 1787–1820 (2021).
La, T.-G. & Le, L. H. Flexible and wearable ultrasound device for medical applications: a review on materials, structural designs, and current challenges. Adv. Mater. Technol. 7, 2100798 (2022).
Jang, H. et al. Graphene e-tattoos for unobstructive ambulatory electrodermal activity sensing on the palm enabled by heterogeneous serpentine ribbons. Nat. Commun. 13, 6004 (2022).
Wang, M. et al. A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 6, 1225–1235 (2022).
Sani, E. S. et al. A stretchable wireless wearable bioelectronic system for multiplexed monitoring and combination treatment of infected chronic wounds. Sci. Adv. 9, eadf7388 (2023).
Heng, W. et al. A smart mask for exhaled breath condensate harvesting and analysis. Science 385, 954–961 (2024).
Kim, T. Y. et al. Smart contact lenses with a transparent silver nanowire strain sensor for continuous intraocular pressure monitoring. ACS Appl. Bio Mater. 4, 4532–4541 (2021).
Nguyen, P. Q. et al. Wearable materials with embedded synthetic biology sensors for biomolecule detection. Nat. Biotechnol. 39, 1366–1374 (2021).
Xu, Y. et al. In-ear integrated sensor array for the continuous monitoring of brain activity and of lactate in sweat. Nat. Biomed. Eng. 7, 1307–1320 (2023).
Kang, T. et al. Penetrative and sustained drug delivery using injectable hydrogel nanocomposites for postsurgical brain tumor treatment. ACS Nano 17, 5435–5447 (2023).
Zhao, X. et al. Permanent fluidic magnets for liquid bioelectronics. Nat. Mater. 23, 703–710 (2024).
Wang, L. et al. Injectable and conductive cardiac patches repair infarcted myocardium in rats and minipigs. Nat. Biomed. Eng. 5, 1157–1173 (2021).
Tang, H. et al. Injectable ultrasonic sensor for wireless monitoring of intracranial signals. Nature 630, 84–90 (2024).
Deng, J. et al. A bioadhesive pacing lead for atraumatic cardiac monitoring and stimulation in rodent and porcine models. Sci. Transl. Med. 16, eado9003 (2024).
Ramezani, M. et al. High-density transparent graphene arrays for predicting cellular calcium activity at depth from surface potential recordings. Nat. Nanotechnol. 19, 504–513 (2024).
Woodington, B. J. et al. Electronics with shape actuation for minimally invasive spinal cord stimulation. Sci. Adv. 7, eabg7833 (2021).
Liu, J. et al. Bioresorbable shape-adaptive structures for ultrasonic monitoring of deep-tissue homeostasis. Science 383, 1096–1103 (2024).
Sun, P. et al. A biodegradable and flexible neural interface for transdermal optoelectronic modulation and regeneration of peripheral nerves. Nat. Commun. 15, 4721 (2024).
Choi, Y. S. et al. Fully implantable and bioresorbable cardiac pacemakers without leads or batteries. Nat. Biotechnol. 39, 1228–1238 (2021).
Wu, H. et al. Accelerated intestinal wound healing via dual electrostimulation from a soft and biodegradable electronic bandage. Nat. Electron. 7, 299–312 (2024).
Stuart, T., Hanna, J. & Gutruf, P. Wearable devices for continuous monitoring of biosignals: challenges and opportunities. APL Bioeng. 6, 021502 (2022).
Halprin, K. M. Epidermal ‘turnover time’ — a re‐examination. Br. J. Dermatol. 86, 14–19 (1972).
Tang, H. et al. Multifunctional conductive hydrogel interface for bioelectronic recording and stimulation. Adv. Healthc. Mater. 13, 2400562 (2024).
Wu, F. et al. Generating dual structurally and functionally skin-mimicking hydrogels by crosslinking cell-membrane compartments. Nat. Commun. 15, 802 (2024).
Gao, Z. et al. Advanced energy harvesters and energy storage for powering wearable and implantable medical devices. Adv. Mater. 36, 2404492 (2024).
Roy, S. et al. Powering solutions for biomedical sensors and implants inside the human body: a comprehensive review on energy harvesting units, energy storage, and wireless power transfer techniques. IEEE Trans. Power Electron. 37, 12237–12263 (2022).
Miyake, T. et al. Direct conductive bonding of silver electrodes on ultrathin polymer films. ACS Appl. Electron. Mater. 6, 7261–7267 (2024).
Li, G. et al. Three-dimensional flexible electronics using solidified liquid metal with regulated plasticity. Nat. Electron. 6, 154–163 (2023).
Park, Y. G., An, H. S., Kim, J. Y. & Park, J. U. High-resolution, reconfigurable printing of liquid metals with three-dimensional structures. Sci. Adv. 5, eaaw2844 (2019).
Tian, X. et al. Implant-to-implant wireless networking with metamaterial textiles. Nat. Commun. 14, 4335 (2023).
Kong, L. et al. Wireless technologies in flexible and wearable sensing: from materials design, system integration to applications. Adv. Mater. 36, 2400333 (2024).
Kim, K. K. et al. A substrate-less nanomesh receptor with meta-learning for rapid hand task recognition. Nat. Electron. 6, 64–75 (2022).
Hu, H. et al. A wearable cardiac ultrasound imager. Nature 613, 667–675 (2023).
Klodell, C. T. Jr et al. Worldwide surgical experience with the Paracor HeartNet cardiac restraint device. J. Thorac. Cardiovasc. Surg. 135, 188–195 (2008).
Acknowledgements
This research was supported by the Institute for Basic Science (IBS-R006-D1 and IBS-R006-A1). Additional funding was provided by the National Research Foundation of Korea (NRF) grant funded by the Korean government (2021R1C1C200440021); the Institute of Information & Communications Technology Planning & Evaluation (IITP)-ITRC (Information Technology Research Center) grant funded by the Korea government (MSIT) (IITP-2025-RS-2024-00438007); and the BK21 FOUR (2120241315235) funded by the Ministry of Education (MOE, Korea), and NRF.
Author information
Authors and Affiliations
Contributions
H.J.K., J.H.K. and S.L. contributed equally to this work. All authors participated in the discussion of content, contributed to the manuscript’s writing and collaboratively provided substantial input and revisions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Materials thanks Tal Dvir, Igor Efimov and Yan Wang for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, H.J., Koo, J.H., Lee, S. et al. Materials design and integration strategies for soft bioelectronics in digital healthcare. Nat Rev Mater 10, 654–673 (2025). https://doi.org/10.1038/s41578-025-00819-w
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41578-025-00819-w
This article is cited by
-
Deformable materials and structures in wearable haptic interfaces
Nature Reviews Materials (2026)
-
AI-native robotic vision systems enabled by in-sensor computing
npj Unconventional Computing (2026)
-
Quantifying the global eco-footprint of wearable healthcare electronics
Nature (2026)
-
Bioelectronic implants built from rolled-up stretchy circuits
Nature (2025)