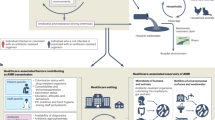
Abstract
The global rise of antimicrobial resistance (AMR) poses a profound threat to human, animal and environmental health. Although antimicrobials have revolutionized modern medicine, their overuse and misuse have accelerated AMR, necessitating urgent, multisectoral action. Antimicrobial stewardship (AMS), a set of coordinated strategies that promote responsible antimicrobial use, has emerged as a key intervention in managing AMR. In this Review, we explore AMS within a One Health framework, emphasizing interconnectedness across sectors. We examine clinical, economic, sociocultural and environmental drivers of antimicrobial use, highlighting disparities between high-income and low-income settings and identifying context-specific challenges to implementation. We also discuss the importance of governance, financing, digital innovation, surveillance and behavioural science in shaping sustainable AMS programmes, and we consider core components, such as policy integration, surveillance of appropriateness, and context-aware interventions. This Review ultimately advocates for equity-focused strategies that better account for structural barriers, support marginalized populations, and ensure global access to high-quality antimicrobials. By aligning political will, funding and scientific innovation, AMS programmes can be scaled effectively to preserve antimicrobial efficacy, mitigate AMR, improve health outcomes, and promote global health security. The paper concludes with key recommendations for embedding AMS across sectors as a sustainable response to AMR.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pandey, A. K. et al. Antimicrobial drug pricing. Commun. Med. 4, 198 (2024).
Kamere, N. et al. Supply chain factors and antimicrobial stewardship. Bull. World Health Organ. 101, 403411 (2023).
World Health Organization. A Study on the Public Health and Socioeconomic Impact of Substandard and Falsified Medical Products (WHO, 2017).
Okeke, I. N. et al. Antimicrobial resistance in developing countries. Part I: recent trends and current status. Lancet Infect. Dis. 5, 481493 (2005).
Zakhour, J. et al. Diagnostic stewardship in infectious diseases: a continuum of antimicrobial stewardship in the fight against antimicrobial resistance. Int. J. Antimicrob. Agents 62, 106816 (2023).
Allegranzi, B. et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet 377, 228241 (2011).
Van Boeckel, T. P. et al. Global trends in antimicrobial use in food animals. Proc. Natl Acad. Sci. USA 112, 56495654 (2015).
Landers, T. F., Cohen, B., Wittum, T. E. & Larson, E. L. A review of antibiotic use in food animals: perspective, policy, and potential. Public Health Rep. 127, 422 (2012).
Larsson, D. G. J. & Flach, C. F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 20, 257269 (2022).
Cameron, A. et al. ‘Mind the Gaps’: stakeholder perspectives on addressing antimicrobial resistance in the environment in the Indian context. Glob. Health Action 18, 2491200 (2025).
Memish, Z. A., Venkatesh, S. & Shibl, A. M. Impact of travel on international spread of antimicrobial resistance. Int. J. Antimicrob. Agents 21, 135142 (2003).
Renwick, M. J., Brogan, D. M. & Mossialos, E. A systematic review and critical assessment of incentive strategies for discovery and development of novel antibiotics. J. Antibiot. 69, 7388 (2016).
McCullough, A. R., Parekh, S., Rathbone, J., Del Mar, C. B. & Hoffmann, T. C. A systematic review of the public’s knowledge and beliefs about antibiotic resistance. J. Antimicrob. Chemother. 71, 2733 (2016).
Dyar, O. J., Huttner, B., Schouten, J. & Pulcini, C. What is antimicrobial stewardship? Clin. Microbiol. Infect. 23, 793798 (2017).
World Health Organization. Antimicrobial Stewardship Programmes in Healthcare Facilities in Low and Middle-income Countries. A Practical Toolkit (WHO, 2019). This resource provides a comprehensive framework for implementing AMS programmes in health-care settings in lower-income and middle-income countries.
Adisasmito, W. B. et al. One Health: a new definition for a sustainable and healthy future. PLoS Pathog. 18, e1010537 (2022).
Food and Agriculture Organization of the United Nations, United Nations Environment Program, World Health Organization & World Organisation for Animal Health. Quadripartite Key Recommendations and Priorities for the 2024 UNGA High-Level Meeting on Antimicrobial Resistance (AMR): A Policy Brief (FAO, UNEP, WHO & WOAH, 2024). This policy brief outlines the key recommendations and priorities of the Quadripartite organizations for addressing AMR at the 2024 UNGA High-Level Meeting and emphasizes the need for a One Health approach to advance global action and collaboration against AMR.
Thursky, K. A. et al. Antimicrobial stewardship in Australia: the role of qualitative research in programme development. JAC Antimicrob. Resist. https://doi.org/10.1093/jacamr/dlab166 (2021). This article outlines the role of implementation science and mixed-methods research used by a One Health programme for AMS in hospitals, primary care, aged care and veterinary clinics.
Hardefeldt, L. Y. & Thursky, K. One Health antimicrobial resistance: stewardship in Australia. Microbiol. Aust. 45, 7982 (2024).
Naghavi, M. et al. Global burden of bacterial antimicrobial resistance 19902021: a systematic analysis with forecasts to 2050. Lancet 404, 11991226 (2024). This systematic analysis quantifies the global burden of bacterial AMR over 30 years (1990 2021) and forecasts its trajectory to 2050, highlighting the urgent need for coordinated interventions to mitigate the growing impact of AMR on public health.
World Health Organization. WHO Fungal Priority Pathogens List to Guide Research Development and Public Health Action (WHO, 2022).
Fisher, M. C. et al. Tackling the emerging threat of antifungal resistance to human health. Nat. Rev. Microbiol. 20, 557571 (2022).
Adamie, B. A. et al. Forecasting the Fallout from AMR: Economic Impacts of Antimicrobial Resistance in Food-Producing Animals: A Report from the EcoAMR Series (WOAH & World Bank, 2024).
Davis, M. D. M. et al. Risk individualisation and moral injury in the treatment of infection as impediments to the tackling of antimicrobial resistance. Health Risk Soc. 26, 222239 (2024).
Lounsbury, O., O’Hara, J., Brent, A. J. & Higham, H. Designing better systems to navigate the sepsis-antimicrobial stewardship tension. Lancet Infect. Dis. https://doi.org/10.1016/s14733099(25)001197 (2025).
Rudd, K. E. et al. Global, regional, and national sepsis incidence and mortality, 19902017: analysis for the global burden of disease study. Lancet 395, 200211 (2020).
World Organisation for Animal Health. Annual Report on Antimicrobial Agents Intended for Use in Animals: 8th Report (WOAH, 2024).
Yin, X., Dudley, E. G., Pinto, C. N. & M’Ikanatha, N. M. Fluoroquinolone sales in food animals and quinolone resistance in nontyphoidal salmonella from retail meats: United States, 2009-2018. J. Glob. Antimicrob. Resist. 29, 163167 (2022).
Goggs, R. et al. Patterns of antimicrobial drug use in veterinary primary care and specialty practice: a 6-year multi-institution study. J. Vet. Intern. Med. 35, 14961508 (2021).
Spurling, G. K. et al. Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: a systematic review. PLoS Med. 7, e1000352 (2010).
Richards, S. et al. Cross-sectional evaluation of a large-scale antimicrobial stewardship trial in Australian companion animal practices. Vet. Rec. 194, e3268 (2024).
European Commission. EC Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on Veterinary Medicinal Products and Repealing Directive 2001/82/EC (EU, 2019).
Carmo, L. P. et al. Veterinary expert opinion on potential drivers and opportunities for changing antimicrobial usage practices in livestock in Denmark, Portugal, and Switzerland. Front. Vet. Sci. 5, 29 (2018).
Carson, C. et al. Ceftiofur-resistant Salmonella enterica serovar Heidelberg of poultry origin — a risk profile using the Codex framework. Epidemiol. Infect. 147, e296 (2019).
Andrés-Lasheras, S., Jelinski, M., Zaheer, R. & McAllister, T. A. Bovine respiratory disease: conventional to culture-independent approaches to studying antimicrobial resistance in North America. Antibiotics 11, 487 (2022).
World Organisation for Animal Health. Monitoring of the Quantities and Usage Patterns of Antimicrobial Agents Used in Food Producing Animals (WOAH, 2023).
Simjee, S. & Ippolito, G. European regulations on prevention use of antimicrobials from January 2022. Braz. J. Vet. Med. 44, e000822 (2022).
Van Boeckel, T. P. et al. Global trends in antimicrobial resistance in animals in low and middle-income countries. Science 365, eaaw1944 (2019).
World Organisation for Animal Health. Annual Report on Antimicrobial Agents Intended for Use in Animals: 7th Report (WOAH, 2023).
United Nations Environment Programme. Bracing for Superbugs: Strengthening Environmental Action in the One Health Response to Antimicrobial Resistance (UNEP, 2023).
Karkman, A., Do, T. T., Walsh, F. & Virta, M. P. J. Antibiotic resistance genes in waste water. Trends Microbiol. 26, 220228 (2018).
Berendonk, T. U. et al. Tackling antibiotic resistance: the environmental framework. Nat. Rev. Microbiol. 13, 310317 (2015).
Lim, A. et al. Exploring awareness of planetary health and antibiotic disposal advice across Australian pharmacies: a mystery shopping expedition. Environ. Chall. 17, 101020 (2024).
World Health Organization. Antimicrobial Resistance: A Manual for Developing National Action Plans (WHO, 2016).
Rizzo, L. et al. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: a review. Sci. Total Environ. 447, 345360 (2013).
Walpole, S. C., Eii, M. N., Lyons, T. & Aldridge, C. Improving antimicrobial use to protect the environment: what is the role of infection specialists? Antibiotics 12, 640 (2023).
World Health Organization. WHO Global Action Plan on Antimicrobial Resistance (WHO, 2015).
United Nations General Assembly. Political declaration of the high-level meeting of the general assembly on antimicrobial resistance (2016).
World Health Organization. WHO bacterial priority pathogens list, 2024: bacterial pathogens of public health importance to guide research, development and strategies to prevent and control antimicrobial resistance (WHO, 2024).
World Health Organization. WHO’s list of medically important antimicrobials: a risk management tool for mitigating antimicrobial resistance due to nonhuman use (WHO, 2024).
Food and Agriculture Organization of the United Nations and World Health Organization. Guidelines for risk analysis of foodborne antimicrobial resistance (FAO, WHO, 2011).
Zanichelli, V. et al. The WHO AWaRe (Access, Watch, Reserve) antibiotic book and prevention of antimicrobial resistance. Bull. World Health Organ. 101, 290296 (2023).
World Health Organization. The WHO AWaRe (Access, Watch, Reserve) Antibiotic Book (WHO, 2022).
World Organisation for Animal Health. Annual Report on Antimicrobial Agents Intended for Use in Animals: 9th Report (WOAH, 2025).
Food and Agriculture Organization of the United Nations. Reduce the need for antimicrobials on farms for sustainable agrifood systems transformation. FAO https://www.fao.org/antimicrobial-resistance/background/fao-role/renofarm/en/ (2025).
Australian Strategic and Technical Advisory Group on Antimicrobial Resistance. Importance Ratings and Summary of Antibacterial Uses in Humans and Animal Health in Australia (Commonwealth of Australia, 2018).
Health Canada. Categorization of Antimicrobial Drugs Based on Importance in Human Medicine (Government of Canada, 2009).
Food Safety Commission Japan. Ranking of the Importance of Antimicrobials Against Bacteria Which Affect Human Health Through Food Commodities (Ministry of Agriculture, Forestry and Fisheries of Japan, 2014).
AntiMicrobial Expert Group. Categorisation of Antibiotics in the European Union (European Medicines Agency, 2019).
Food and Drug Administration. Guidance for Industry #152: Evaluating the Safety of Antimicrobial New Animal Drugs with Regard to their Microbiological Effects on Bacteria of Human Health Concern (FDA, 2003).
Hardefeldt, L. Y. et al. Antimicrobial stewardship in companion animal practice: an implementation trial in 135 general practice veterinary clinics. JAC Antimicrob. Resist. 4, dlac015 (2022).
Hopman, N. E. M. et al. Implementation and evaluation of an antimicrobial stewardship programme in companion animal clinics: a stepped-wedge design intervention study. PLoS ONE 14, e0225124 (2019).
Morgans, L. C. et al. A participatory farmer-led approach to changing practices around antimicrobial use on UK farms. J. Dairy Sci. 104, 22122230 (2021).
European Medicines Agency & European Surveillance of Veterinary Antimicrobial Consumption. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2022 (European Medicines Agency, 2023).
Charani, E. et al. An analysis of existing national action plans for antimicrobial resistance gaps and opportunities in strategies optimising antibiotic use in human populations. Lancet Glob. Health 11, e466e474 (2023). This article provides an in-depth analysis of national action plans and highlights the challenges between LMICS and HICs in the operationalization of national action plans.
Harun, M. G. D. et al. Barriers, facilitators, perceptions and impact of interventions in implementing antimicrobial stewardship programs in hospitals of low-middle and middle countries: a scoping review. Antimicrob. Resist. Infect. Control 13, 8 (2024).
Lewnard, J. A. et al. Burden of bacterial antimicrobial resistance in low-income and middle-income countries avertible by existing interventions: an evidence review and modelling analysis. Lancet 403, 24392454 (2024). This study evaluates the burden of bacterial AMR in low-income and middle-income countries, demonstrating that its reduction is achievable with existing interventions, and provides a framework for evidence-based AMR control strategies.
Patel, J. et al. Measuring the global response to antimicrobial resistance, 202021: a systematic governance analysis of 114 countries. Lancet Infect. Dis. 23, 706718 (2023). This study is a systematic governance analysis of the responses of 114 countries to AMR, which highlights progress, gaps to be addressed, and the need for stronger global coordination and accountability in tackling AMR.
Sefah, I. A., Chetty, S., Yamoah, P., Godman, B. & Bangalee, V. An assessment of the current level of implementation of the core elements of antimicrobial stewardship programs in public hospitals in Ghana. Hosp. Pharm. 59, 367377 (2024).
Murray, J. L. et al. Drivers of inappropriate use of antimicrobials in South Asia: a systematic review of qualitative literature. PLoS Glob. Public Health 4, e0002507 (2024).
Büchler, A. C. et al. Challenges and success stories of the implementation of infection control and antimicrobial stewardship strategies: proceedings of the 5th Global Ministerial Summit on Patient Safety, 2023. Antimicrob. Resist. Infect. Control 13, 16 (2024).
Restrepo-Arbeláez, N., Garcia-Betancur, J. C., Pallares, C. J. & Villegas, M. V. Antimicrobial stewardship programs in Latin America and the Caribbean: a story of perseverance, challenges, and goals. Antibiotics 12, 1342 (2023).
Pallares, C. J. et al. Antimicrobial stewardship programs in seven Latin American countries: facing the challenges. BMC Infect. Dis. 23, 463 (2023).
Compri, M. et al. White paper: Bridging the gap between surveillance data and antimicrobial stewardship in the animal sector — practical guidance from the JPIAMR ARCH and COMBACTE-MAGNET EPI-Net networks. J. Antimicrob. Chemother. 75, ii52–ii66 (2020).
Damborg, P. et al. ENOVAT: the European Network for Optimization of Veterinary Antimicrobial Treatment. Open Res. Eur. 4, 170 (2024).
Rees, G. M., Bard, A. & Reyher, K. K. Designing a national veterinary prescribing champion programme for Welsh veterinary practices: the Arwain Vet Cymru Project. Antibiotics 10, 253 (2021).
Responsible Use of Medicines Alliance (RUMA) Companion Animal and Equine (CA&E). Report on 2023 Veterinary Antibiotic Amnesty (RUMA CA&E, 2024).
Yusuf, H., Hillman, A., Stegeman, J. A., Cameron, A. & Badger, S. Expanding access to veterinary clinical decision support in resource-limited settings: a scoping review of clinical decision support tools in medicine and antimicrobial stewardship. Front. Vet. Sci. 11, 1349188 (2024).
Craig, J. et al. Behavior-change interventions to improve antimicrobial stewardship in human health, animal health, and livestock agriculture: a systematic review. PLoS Glob. Public Health 3, e0001526 (2023). This systematic review examines behaviour-change interventions aimed at improving AMS across human health, animal health and livestock agriculture, and it stresses the importance of a multifaceted approach that integrates education, policy and social incentives.
International Centre for Antimicrobial Resistance Solutions. Financing Antimicrobial Resistance Interventions: Optimizing Current and New Funding Mechanisms for Mitigation. Summary Brief from a High-level Side Event During the World Health Assembly (ICARS, 2024).
Raboisson, D., Ferchiou, A., Sans, P., Lhermie, G. & Dervillé, M. The economics of antimicrobial resistance in veterinary medicine: optimizing societal benefits through mesoeconomic approaches from public and private perspectives. One Health 10, 100145 (2020).
Barlam, T. F. et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 62, e5177 (2016).
Australian Commission on Safety and Quality in Health Care. Antimicrobial Stewardship in Australian Health Care (ACSQHC, 2023).
World Health Organization. Antimicrobial Stewardship Interventions: A Practical Guide (WHO, 2021).
World Health Organization. Status on National Core Elements for Antimicrobial Stewardship Programmes in the WHO African Region (WHO, 2024).
National Institute for Health and Care Excellence. Antimicrobial Stewardship: Systems and Processes for Effective Antimicrobial Medicine Use (WHO, 2015).
European Commission. EU Guidelines for the Prudent Use of Antimicrobials in Human Health (2017/C 212/01) (Official Journal of the European Union, 2017).
American Veterinary Medical Association. Antimicrobial Stewardship Definition and Core Principles (AVMA, 2018).
Llor, C. & Bjerrum, L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 5, 229241 (2014).
Samreen, Ahmad, I., Malak, H. A. & Abulreesh, H. H. Environmental antimicrobial resistance and its drivers: a potential threat to public health. J. Glob. Antimicrob. Resist. 27, 101111 (2021).
Chang, F. Y. et al. Gaps in antimicrobial stewardship programmes in Asia: a survey of 10 countries. JAC Antimicrob. Resist. 4, dlac117 (2022).
Pulcini, C. et al. Developing core elements and checklist items for global hospital antimicrobial stewardship programmes: a consensus approach. Clin. Microbiol. Infect. 25, 2025 (2019).
Mendelson, M., Morris, A. M., Thursky, K. & Pulcini, C. How to start an antimicrobial stewardship programme in a hospital. Clin. Microbiol. Infect. 26, 447453 (2020). This paper provides a roadmap for implementation of complete AMS programmes in the hospital setting.
Bhowmick, T., Abdul Azim, A., Narayanan, N. & Kaye, K. Traits and skills of effective leaders in antimicrobial stewardship. Antimicrob. Stewardship Healthc. Epidemiol. 3, e222 (2023).
Ashiru-Oredope, D. et al. Development and implementation of an antimicrobial stewardship checklist in sub-Saharan Africa: a co-creation consensus approach. Healthcare 10, 1706 (2022).
Pierce, J. et al. Global antimicrobial stewardship with a focus on low and middle-income countries. Int. J. Infect. Dis. 96, 621629 (2020).
Pauwels, I. et al. Implementation of hospital antimicrobial stewardship programmes in low- and middle-income countries: a qualitative study from a multi-professional perspective in the Globa-lPPS network. Antimicrob. Resist. Infect. Control 14, 26 (2025).
Pulcini, C. et al. Human resources estimates and funding for antibiotic stewardship teams are urgently needed. Clin. Microbiol. Infect. 23, 785787 (2017).
Greene, M. H., Nesbitt, W. J. & Nelson, G. E. Antimicrobial stewardship staffing: how much is enough? Infect. Control Hospital Epidemiol. 41, 102112 (2020).
MacDougall, C. & Polk, R. E. Antimicrobial stewardship programs in health care systems. Clin. Microbiol. Rev. 18, 638656 (2005).
Nampoothiri, V. et al. What does antimicrobial stewardship look like where you are? Global narratives from participants in a massive open online course. JAC Antimicrob. Resist. 4, dlab186 (2022).
Nampoothiri, V. et al. Evolution of pharmacist roles in antimicrobial stewardship: a 20-year systematic review. Int. J. Infect. Dis. 151, 107306 (2024). This systematic review examines the evolving roles of pharmacists in AMS over the past 20 years and highlights the critical contributions of pharmacists to optimizing antimicrobial use and improving patient outcomes.
OkedoAlex, I. N., Akamike, I. C., Iyamu, I. & Umeokonkwo, C. D. Pattern of antimicrobial prescription in Africa: a systematic review of point prevalence surveys. Pan Afr. Med. J. 45, 67 (2023).
Satterfield, J., Miesner, A. R. & Percival, K. M. The role of education in antimicrobial stewardship. J. Hospital Infect. 105, 130141 (2020).
Alves, J. et al. Establishing core competencies for antimicrobial stewardship teams: a consensus development using the modified Delphi technique — an European Society of Clinical Microbiology and Infectious Diseases Study Group for Antimicrobial Stewardship position paper. Clin. Microbiol. Infect. https://doi.org/10.1016/j.cmi.2025.04.035 (2025)
Yau, J. W., Thor, S. M., Tsai, D., Speare, T. & Rissel, C. Antimicrobial stewardship in rural and remote primary health care: a narrative review. Antimicrob. Resist. Infect. Control 10, 105 (2021).
Dellit, T. H. et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin. Infect. Dis. 44, 159177 (2007).
Dyar, O. J., Tebano, G. & Pulcini, C. Managing responsible antimicrobial use: perspectives across the healthcare system. Clin. Microbiol. Infect. 23, 441447 (2017).
Rupali, P. et al. Impact of an antimicrobial stewardship intervention in India: evaluation of post-prescription review and feedback as a method of promoting optimal antimicrobial use in the intensive care units of a tertiary-care hospital. Infect. Control Hospital Epidemiol. 40, 512519 (2019).
Shallal, A. et al. The impact of a post-prescription review and feedback antimicrobial stewardship program in Lebanon. Antibiotics 11, 642 (2022).
Davey, P. et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst. Rev. 9, CD003543 (2017). This is a seminal systematic review that provides evidence for the core activities for AMS programmes in hospitals, specifically providing high-certainty evidence that AMS interventions (both enablement and restriction) are effective in increasing compliance with AMS policy and reducing duration of treatment, and that lower use of antibiotics probably does not increase mortality and probably reduces length of stay.
Paulson, C. M. et al. Impact of a systematic pharmacist-initiated antibiotic time-out intervention for hospitalized adults. J. Pharm. Pract. 35, 388395 (2022).
Mishima, Y. et al. Impact of antibiotic timeouts in multidisciplinary ICU rounds for antimicrobial stewardship program on patient survival: a controlled before-and-after study. Crit. Care Explor. 5, e0837 (2023).
Sarraf-Yazdi, S. et al. A 9-year retrospective review of antibiotic cycling in a surgical intensive care unit. J. Surg. Res. 176, e7378 (2012).
Timbrook, T. T. et al. The effect of molecular rapid diagnostic testing on clinical outcomes in bloodstream infections: a systematic review and meta-analysis. Clin. Infect. Dis. 64, 1523 (2017).
Scandrett, K. et al. Evidence Review for Rapid Tests to Inform Triage and Antibiotic Prescribing Decisions: Suspected Acute Respiratory Infection in Over 16s: Assessment at First Presentation and Initial Management: Evidence Review B (National Institute for Health and Care Excellence, 2023).
Nathwani, D. et al. Value of hospital antimicrobial stewardship programs [ASPs]: a systematic review. Antimicrob. Resist. Infect. Control 8, 35 (2019). This systematic review demonstrates the clinical and economic impacts of hospital AMS programmes, but with limited data from lower-income and middle-income countries.
Moehring, R. W. et al. Expert consensus on metrics to assess the impact of patient-level antimicrobial stewardship interventions in acute-care settings. Clin. Infect. Dis. 64, 377383 (2017).
Aljefri, D. M., Christensen, A. B., Gibson, A. K. & Postelnick, M. Role of antimicrobial stewardship programs in decreasing hospital-associated Clostridium difficile infections. Curr. Treat. Options Infect. Dis. 10, 270280 (2018).
Doron, S. & Davidson, L. E. Antimicrobial stewardship. Mayo Clin. Proc. 86, 11131123 (2011).
Seijas-Pereda, L. et al. Global dynamics of gastrointestinal colonisations and antimicrobial resistance: insights from international travellers to low and middle-income countries. Tropical Med. Infect. Dis. 9, 182 (2024).
Morris, A. M. Antimicrobial stewardship programs: appropriate measures and metrics to study their impact. Curr. Treat. Options Infect. Dis. 6, 101112 (2014).
UK Government. UK Veterinary Antimicrobial Resistance and Sales Surveillance 2023 (Gov.UK, 2023).
Food and Agriculture Organization of the United Nations. InFARM system. FAO https://www.fao.org/antimicrobial-resistance/resources/infarm-system/en/ (2025).
Zay Ya, K., Win, P. T. N., Bielicki, J., Lambiris, M. & Fink, G. Association between antimicrobial stewardship programs and antibiotic use globally: a systematic review and meta-analysis. JAMA Netw. Open6, e2253806 (2023). The article provides robust evidence demonstrating that AMS programmes substantially reduce antimicrobial consumption globally and addresses the effectiveness of AMS programmes across diverse health-care settings, including high-income countries and low-income and middle-income countries, where there is a need for adapted strategies to overcome operational and financial barriers.
Hardefeldt, L. Y. et al. Antimicrobial labelling in Australia: a threat to antimicrobial stewardship? Aust. Vet. J. 96, 151154 (2018).
Rachina, S. et al. Assessment of antimicrobial consumption in multi-field hospitals with pediatric inpatients: conventional vs. novel pediatric-adjusted methodologies. Antibiotics 12, 1162 (2023).
Kritsotakis, E. I. & Gikas, A. Surveillance of antibiotic use in hospitals: methods, trends and targets. Clin. Microbiol. Infect. 12, 701704 (2006).
Collineau, L. et al. Guidance on the selection of appropriate indicators for quantification of antimicrobial usage in humans and animals. Zoonoses Public Health 64, 165184 (2017).
Umair, M. et al. Measuring antimicrobial use needs global harmonization. Glob. Chall. 5, 2100017 (2021).
Stanic Benic, M. et al. Metrics for quantifying antibiotic use in the hospital setting: results from a systematic review and international multidisciplinary consensus procedure. J. Antimicrob. Chemother. 73, vi50vi58 (2018).
Polk, R. E., Fox, C., Mahoney, A., Letcavage, J. & MacDougall, C. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin. Infect. Dis. 44, 664670 (2007).
Dalton, B. R., Sabuda, D. M., Bresee, L. C. & Conly, J. M. Assessment of antimicrobial utilization metrics: days of therapy versus defined daily doses and pharmacy dispensing records versus nursing administration data. Infect. Control Hospital Epidemiol. 36, 688694 (2015).
Först, G. et al. Validation of adapted daily dose definitions for hospital antibacterial drug use evaluation: a multicentre study. J. Antimicrob. Chemother. 72, 29312937 (2017).
Gagliotti, C. et al. Hospital statistics for antibiotics: defined versus prescribed daily dose. Infection 42, 869873 (2014).
Pucken, V. B. et al. Antimicrobial consumption: comparison of three different data collection methods. Prev. Vet. Med. 186, 105221 (2021).
Moreno, M. A., Collineau, L. & Carson, C. A. Editorial: Antimicrobial usage in companion and food animals: methods, surveys and relationships with antimicrobial resistance in animals and humans. Front. Vet. Sci. 7, 728267 (2020).
Werner, N., McEwen, S. & Kreienbrock, L. Monitoring antimicrobial drug usage in animals: methods and applications. Microbiol. Spectr. https://doi.org/10.1128/microbiolspec.arba00152017 (2018).
Ching, C., Zaman, M. H. & Wirtz, V. J. Evaluation of surveillance strategies of antimicrobial consumption in animals. Antibiotics 13, 505 (2024).
Ajulo, S. & Awosile, B. Global Antimicrobial Resistance and Use Surveillance System (GLASS 2022): investigating the relationship between antimicrobial resistance and antimicrobial consumption data across the participating countries. PLoS ONE 19, e0297921 (2024). This article investigates the relationship between AMR and antimicrobial consumption using data from GLASS 2022 and emphasizes the critical need for global strategies to curb antimicrobial overuse and improve stewardship to combat the growing threat of AMR.
World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022 (WHO, 2022).
Slama, T. G. et al. A clinician’s guide to the appropriate and accurate use of antibiotics: the Council for Appropriate and Rational Antibiotic Therapy (CARAT) criteria. Am. J. Med. 118, 1s6s (2005).
Alm, R. A. & Lahiri, S. D. Narrow-spectrum antibacterial agents — benefits and challenges. Antibiotics 9, 418 (2020).
Fu, M. et al. Appropriate use of antibiotics for acute respiratory infections at primary healthcare facilities in China: a nationwide cross-sectional study from 2017 to 2019. Lancet Reg. Health West. Pac. https://doi.org/10.1016/j.lanwpc.2023.100880 (2023)
Cantudo-Cuenca, M. R., Jimenez-Morales, A. & la Plata, J. E. M. Pharmacist-driven antimicrobial stewardship program in a long-term care facility by assessment of appropriateness. Eur. Geriatr. Med. 13, 13571364 (2022).
Sassine, J., Skevaki, C. & Chemaly, R. F. Infections in immunocompromised hosts: progress made and challenges ahead. Clin. Microbiol. Infect. https://doi.org/10.1016/j.cmi.2024.10.017 (2024).
Shafiq, N. et al. Shortage of essential antimicrobials: a major challenge to global health security. BMJ Glob. Health 6, e006961 (2021).
Markussen, D. L. et al. Determinants of non-adherence to antibiotic treatment guidelines in hospitalized adults with suspected community-acquired pneumonia: a prospective study. Antimicrob. Resist. Infect. Control 13, 140 (2024).
Lesho, E. P. & Laguio-Vila, M. The slow-motion catastrophe of antimicrobial resistance and practical interventions for all prescribers. Mayo Clin. Proc. 94, 10401047 (2019).
Pandey, A. K. et al. A systematic review of antibiotic drug shortages and the strategies employed for managing these shortages. Clin. Microbiol. Infect. https://doi.org/10.1016/j.cmi.2024.09.023 (2024). This systematic review investigates global antibiotic drug shortages, and the strategies used to manage them, while emphasizing the need for proactive policies and systems to ensure a stable supply of essential antibiotics.
Australian Commission on Safety and Quality in Health Care. National Safety and Quality Health Service Standards 2nd edn (ACSQHC, 2021).
Australian Government. Australia’s National Antimicrobial Resistance Strategy — 2020 and Beyond (Australian Government, 2019).
Royal Melbourne Hospital and the National Centre for Antimicrobial Stewardship. Antimicrobial Prescribing Practice in Australian Hospitals. Results of the 2022 Hospital National Antimicrobial Prescribing Survey (Australian Government, 2024).
McMullan, B. J. et al. Antibiotic appropriateness and guideline adherence in hospitalized children: results of a nationwide study. J. Antimicrob. Chemother. 75, 738746 (2020).
McMullan, B. et al. Antibiotic prescribing in neonatal sepsis: an Australian nationwide survey. BMJ Paediatr. Open 4, e000643 (2020).
Douglas, A. P. et al. Quality of inpatient antimicrobial use in hematology and oncology patients. Infect. Control Hospital Epidemiol. 42, 12351244 (2021).
Cuningham, W. et al. Antimicrobial stewardship in remote primary healthcare across northern Australia. Peer J. 8, e9409 (2020).
World Health Organization. WHO Methodology for Point Prevalence Survey on Antibiotic Use in Hospitals (WHO, 2019).
James, R. et al. The feasibility and generalizability of assessing the appropriateness of antimicrobial prescribing in hospitals: a review of the Australian National Antimicrobial Prescribing Survey. JAC Antimicrob. Resist. 4, dlac012 (2022).
Jamaluddin, N. A. H. et al. Assessment of antimicrobial prescribing patterns, guidelines compliance, and appropriateness of antimicrobial prescribing in surgical-practice units: point prevalence survey in Malaysian teaching hospitals. Front. Pharmacol. 15, 1381843 (2024).
Simon, M. et al. Selection of proxy indicators estimating the appropriateness of antibiotic prescriptions in general practice: a national consensus procedure in France. JAC Antimicrob. Resist. 6, dlae059 (2024).
Funiciello, E. et al. Identifying AWaRe indicators for appropriate antibiotic use: a narrative review. J. Antimicrob. Chemother. https://doi.org/10.1093/jac/dkae370 (2024).
European Sales and Use of Antimicrobials for Veterinary Medicine. Annual Surveillance Report for 2023 (ESUAvet, 2025).
Mendelson, M. et al. Antimicrobial resistance and the great divide: inequity in priorities and agendas between the Global North and the Global South threatens global mitigation of antimicrobial resistance. Lancet Glob. Health 12, e516e521 (2024).
Charani, E. et al. Navigating sociocultural disparities in relation to infection and antibiotic resistance — the need for an intersectional approach. JAC Antimicrob. Resist. 3, dlab123 (2021).
Cohn, J. et al. Accelerating antibiotic access and stewardship: a new model to safeguard public health. Lancet Infect. Dis. 24, e584e590 (2024).
Keenan, K. et al. Intersecting social and environmental determinants of multidrug-resistant urinary tract infections in East Africa beyond antibiotic use. Nat. Commun. 15, 9418 (2024).
Shutt, A. E. et al. The intersection of the social determinants of health and antimicrobial resistance in human populations: a systematic review. BMJ Glob. Health https://doi.org/10.1136/bmjgh2024017389 (2025).
Ong, S. W. X. et al. Reporting of sociodemographic characteristics of trial participants in infectious diseases clinical trials systematic review. Clin. Microbiol. Infect. https://doi.org/10.1016/j.cmi.2025.04.030 (2025).
Crenshaw, K. C. Mapping the margins: intersectionality, identity politics, and violence against women of color. Stanf. Law Rev. 43, 12411299 (1991).
Guerra, P., De Maio, F. & Streed, C. G. Jr. Facing political attacks on medical education the future of diversity, equity, and inclusion in medicine. N. Engl. J. Med. 392, 941944 (2025).
Vaughn, V. M. et al. Pharmacist gender and physician acceptance of antibiotic stewardship recommendations: an analysis of the reducing overuse of antibiotics at discharge home intervention. Infect. Control Hospital Epidemiol. 44, 570577 (2023).
Charani, E. et al. Understanding the determinants of antimicrobial prescribing within hospitals: the role of “prescribing etiquette”. Clin. Infect. Dis. 57, 188196 (2013).
Charani, E. et al. Power Relations in Optimisation of Therapies and Equity in Access to Antibiotics (PROTEA) study: investigating the intersection of socio-economic and cultural drivers on antimicrobial resistance (AMR) and its influence on healthcare access and health-providing behaviours in India and South Africa. Wellcome Open Res. https://doi.org/10.12688/wellcomeopenres.20193.1 (2024).
World Health Organization. Addressing Gender Inequalities in National Action Plans on Antimicrobial Resistance: Guidance to Complement the People-centred Approach (WHO, 2024).
Pouly, E., Coppry, M., Rogues, A. M. & Dumartin, C. Systematic review of factors promoting behaviour change toward antibiotic use in hospitals. Clin. Microbiol. Infect. 28, 911919 (2022).
Duncan, E. M. et al. A behavioural approach to specifying interventions: what insights can be gained for the reporting and implementation of interventions to reduce antibiotic use in hospitals? J. Antimicrob. Chemother. 75, 13381346 (2020).
Borek, A. J., Santillo, M., Wanat, M., Butler, C. C. & Tonkin-Crine, S. How can behavioural science contribute to qualitative research on antimicrobial stewardship in primary care? JAC Antimicrob. Resist. 4, dlac007 (2022). This article explains how behaviour science can improve understanding of the decision-making of health-care professionals regarding antimicrobial prescribing, and enhance AMS qualitative research.
Verliat, F. et al. An efficient cephalosporin stewardship programme in French swine production. Vet. Med. Sci. 7, 432439 (2021).
Cane, J., O’Connor, D. & Michie, S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement. Sci. 7, 37 (2012).
Jones, L. F. et al. Qualitative study using interviews and focus groups to explore the current and potential for antimicrobial stewardship in community pharmacy informed by the Theoretical Domains Framework. BMJ Open 8, e025101 (2018).
Dowson, L. et al. Antimicrobial stewardship near the end of life in aged care homes. Am. J. Infect. Control 48, 688694 (2020).
Ierano, C. et al. Influences on surgical antimicrobial prophylaxis decision making by surgical craft groups, anaesthetists, pharmacists and nurses in public and private hospitals. PLoS ONE 14, e0225011 (2019).
Jinks, T. et al. Opportunities to enhance diagnostic testing and antimicrobial stewardship: a qualitative multinational survey of healthcare professionals. Infect. Dis. Ther. 13, 16211637 (2024).
Van Dort, B. A. et al. A tale of 2 digital hospitals: a qualitative study of antimicrobial stewardship teams. Br. J. Clin. Pharmacol. 90, 11521161 (2024).
Bremmer, D. N., Trienski, T. L., Walsh, T. L. & Moffa, M. A. Role of technology in antimicrobial stewardship. Med. Clin. 102, 955963 (2018).
Rittmann, B. & Stevens, M. P. Clinical decision support systems and their role in antibiotic stewardship: a systematic review. Curr. Infect. Dis. Rep. 21, 29 (2019).
Van Dort, B. A., Carland, J. E., Penm, J., Ritchie, A. & Baysari, M. T. Digital interventions for antimicrobial prescribing and monitoring: a qualitative meta-synthesis of factors influencing user acceptance. J. Am. Med. Inform. Assoc. 29, 17861796 (2022).
Peiffer-Smadja, N. et al. Machine learning for clinical decision support in infectious diseases: a narrative review of current applications. Clin. Microbiol. Infect. 26, 584595 (2020). This article reviews the application of machine learning, highlighting the potential to enhance diagnostic accuracy, predict treatment outcomes, and improve AMS, while emphasizing the limited development and evaluation in low-income and middle-income countries, and advocating for inclusive data integration and context-specific applications, to ensure equity in health-care innovations.
Rawson, T. M. et al. A systematic review of clinical decision support systems for antimicrobial management: are we failing to investigate these interventions appropriately? Clin. Microbiol. Infect. 23, 524532 (2017).
Kuper, K. M., Nagel, J. L., Kile, J. W., May, L. S. & Lee, F. M. The role of electronic health record and “add-on” clinical decision support systems to enhance antimicrobial stewardship programs. Infect. Control Hospital Epidemiol. 40, 501511 (2019).
Van Dort, B. A., Penm, J., Ritchie, A. & Baysari, M. T. The impact of digital interventions on antimicrobial stewardship in hospitals: a qualitative synthesis of systematic reviews. J. Antimicrob. Chemother. 77, 18281837 (2022). This article provides a synthesis of the effectiveness of digital interventions in improving antimicrobial prescribing and monitoring, demonstrating evidence of reductions in antimicrobial use and improvements in prescribing appropriateness, while emphasizing the need for tailored implementation strategies and continuous evaluation to maximize the impact of digital health technologies in AMS.
Rawson, T. M. et al. Optimizing antimicrobial use: challenges, advances and opportunities. Nat. Rev. Microbiol. 19, 747758 (2021).
Marra, A. R., Nori, P., Langford, B. J., Kobayashi, T. & Bearman, G. Brave new world: leveraging artificial intelligence for advancing healthcare epidemiology, infection prevention, and antimicrobial stewardship. Infect. Control Hospital Epidemiol. 44, 19091912 (2023).
Pinto-de-Sá, R., Sousa-Pinto, B. & Costa-de-Oliveira, S. Brave new world of artificial intelligence: its use in antimicrobial stewardship — a systematic review. Antibiotics https://doi.org/10.3390/antibiotics13040307 (2024).
Giacobbe, D. R. et al. Artificial intelligence and prescription of antibiotic therapy: present and future. Expert Rev. Antiinfect. Ther. https://doi.org/10.1080/14787210.2024.2386669 (2024).
Chang, A. & Chen, J. H. BSAC Vanguard Series: artificial intelligence and antibiotic stewardship. J. Antimicrob. Chemother. 77, 12161217 (2022).
Pennisi, F., Pinto, A., Ricciardi, G. E., Signorelli, C. & Gianfredi, V. Artificial intelligence in antimicrobial stewardship: a systematic review and meta-analysis of predictive performance and diagnostic accuracy. Eur. J. Clin. Microbiol. Infect. Dis. 44, 463513 (2025).
Pennisi, F., Pinto, A., Ricciardi, G. E., Signorelli, C. & Gianfredi, V. The role of artificial intelligence and machine learning models in antimicrobial stewardship in public health: a narrative review. Antibiotics 14, 134 (2025).
Harandi, H. et al. Artificial intelligence-driven approaches in antibiotic stewardship programs and optimizing prescription practices: a systematic review. Artif. Intell. Med. 162, 103089 (2025).
Hur, B., Hardefeldt, L. Y., Verspoor, K. M., Baldwin, T. & Gilkerson, J. R. Evaluating the dose, indication and agreement with guidelines of antimicrobial use in companion animal practice with natural language processing. JAC Antimicrob. Resist. 4, dlab194 (2022).
Hur, B., Hardefeldt, L. Y., Verspoor, K., Baldwin, T. & Gilkerson, J. R. Using natural language processing and VetCompass to understand antimicrobial usage patterns in Australia. Aust. Vet. J. 97, 298300 (2019).
Hur, B., Baldwin, T., Verspoor, K., Hardefeldt, L. & Gilkerson, J. Domain adaptation and instance selection for disease syndrome classification over veterinary clinical notes. in Proc. 19th SIGBioMed Workshop on Biomedical Language Processing (Association for Computational Linguistics, 2020).
Khurana, M. P. et al. Mitigating antimicrobial resistance (AMR) using implementation research: a development funder’s approach. JAC Antimicrob. Resist. 5, dlad031 (2023).
World Health Organization, Food and Agriculture Organization of the United Nations, United Nations Environment Programme & World Organisation for Animal Health. The Quadripartite Joint Secretariat on AMR Progress Report: Coordinating and Leading the Global One Health Response to Antimicrobial Resistance (WHO, 2024).
Acknowledgements
The authors thank C. Chen, J. Maleki, Z. Rashidzada and J. Wen for their helpful comments in providing structure and scope for this publication.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Microbiology thanks Afreenish Amir, Maddalena Giannella and Adrian Muwonge for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Antimicrobial Resistance Country Self-Assessment Survey (TrACSS): http://www.amrcountryprogress.org/
Antimicrobial Use and Resistance in Australia (AURA): http://www.safetyandquality.gov.au/our-work/antimicrobial-resistance/antimicrobial-use-and-resistance-australia-aura
Danish Integrated Antimicrobial Resistance Monitoring and Research Program: http://www.danmap.org
European Surveillance of Antimicrobial Consumption Network: http://www.ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/esac-net
Global-PPS: http://www.global-pps.com
Hospital National Antimicrobial Prescribing Survey (Hospital NAPS): http://naps.org.au/
Multi-Partner Trust Fund: http://mptf.undp.org/fund/amr00
National Healthcare Safety Network: http://www.cdc.gov/nhsn/index.html
National prescribing guidelines: http://www.tg.org.au/
Swedish Strategic Program for the Rational Use of Antibiotic Agents and Surveillance of Resistance: http://strama.se/
UK AID Fleming Fund programme: http://www.flemingfund.org/
World Health Organization AWaRe (Access, Watch, Reserve) classification: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.04
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
James, R., Hardefeldt, L.Y., Ierano, C. et al. Antimicrobial stewardship from a One Health perspective. Nat Rev Microbiol 24, 146–162 (2026). https://doi.org/10.1038/s41579-025-01233-3
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41579-025-01233-3