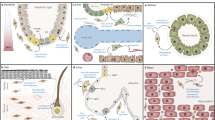
Abstract
Fibroblasts are a special type of interstitial cell that has an essential role in maintaining the structural framework of tissues and organs. In response to injury, fibroblasts are activated and produce large amounts of extracellular matrix proteins. Fibroblast activation has a crucial role in tissue repair and wound healing. However, uncontrolled and persistent activation of fibroblasts ultimately leads to fibrotic diseases of organs such as the kidney, liver, lung and heart. Activated fibroblasts predominantly originate from the local activation and expansion of resident fibroblasts and pericytes. A diverse array of extracellular cues, including soluble factors, extracellular vesicles, matricellular proteins and mechanical stiffness, induce fibroblast activation after tissue injury. Fibroblast activation primarily takes place in the fibrogenic niche, a unique tissue microenvironment in which fibroblasts interact with injured parenchymal cells, inflammatory cells and endothelial cells. The fates of activated fibroblasts, including apoptosis, senescence, dedifferentiation and lineage reprogramming, determine the outcome of tissue repair and organ fibrosis. Potential therapeutic strategies for fibrotic diseases include disrupting the formation of the fibrogenic niche, inhibiting fibroblast activation, promoting fibroblast depletion, accelerating fibrosis resolution or promoting tissue repair and regeneration.
Key points
-
Fibroblast activation is an evolutionarily conserved response to tissue injury. Dysregulated activation of fibroblasts results in excessive production of extracellular matrix (ECM) proteins, leading to tissue scarring in various organs.
-
A diverse array of extracellular stimuli, including soluble factors, extracellular vesicles, matricellular proteins and mechanical cues, induce fibroblast activation after tissue injury. The TWA cycle, consisting of TGFβ, Wnt and angiotensin II, constitutes the core signalling network that drives fibrosis in various organs.
-
Activation of fibroblasts primarily takes place in the fibrogenic niche, a specialized tissue microenvironment in which they interact with injured parenchymal cells, macrophages and endothelial cells via diverse mediators.
-
Following tissue injury, the size of the fibroblast population is controlled not only by fibroblast activation but also by the speed of resolution of fibrosis; the fates of activated fibroblasts include apoptosis, senescence, dedifferentiation and reprogramming.
-
Targeting fibroblasts could be an effective therapeutic strategy for organ fibrosis. Approaches that disrupt the formation of the fibrogenic niche, inhibit fibroblast activation or promote fibroblast depletion are promising strategies for ameliorating fibrotic lesions in various organs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Rockey, D. C., Bell, P. D. & Hill, J. A. Fibrosis — a common pathway to organ injury and failure. N. Engl. J. Med. 372, 1138–1149 (2015).
Lurje, I., Gaisa, N. T., Weiskirchen, R. & Tacke, F. Mechanisms of organ fibrosis: emerging concepts and implications for novel treatment strategies. Mol. Asp. Med. 92, 101191 (2023).
Wynn, T. A. Fibrotic disease and the TH1/TH2 paradigm. Nat. Rev. Immunol. 4, 583–594 (2004).
Zhou, D. et al. Early activation of fibroblasts is required for kidney repair and regeneration after injury. FASEB J. 33, 12576–12587 (2019).
Gomes, R. N., Manuel, F. & Nascimento, D. S. The bright side of fibroblasts: molecular signature and regenerative cues in major organs. NPJ Regen. Med. 6, 43 (2021).
Yuan, Q., Tan, R. J. & Liu, Y. Myofibroblast in kidney fibrosis: origin, activation, and regulation. Adv. Exp. Med. Biol. 1165, 253–283 (2019).
Huang, R., Fu, P. & Ma, L. Kidney fibrosis: from mechanisms to therapeutic medicines. Signal. Transduct. Target. Ther. 8, 129 (2023).
Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 7, 684–696 (2011).
Kisseleva, T. & Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 18, 151–166 (2021).
Fu, H. et al. Tenascin-C is a major component of the fibrogenic niche in kidney fibrosis. J. Am. Soc. Nephrol. 28, 785–801 (2017).
Liu, X. et al. Tubule-derived exosomes play a central role in fibroblast activation and kidney fibrosis. Kidney Int. 97, 1181–1195 (2020).
Kuppe, C. et al. Decoding myofibroblast origins in human kidney fibrosis. Nature 589, 281–286 (2021).
Biasin, V. et al. PDGFRα and αSMA mark two distinct mesenchymal cell populations involved in parenchymal and vascular remodeling in pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 318, L684–L697 (2020).
Mayr, C. H. et al. Sfrp1 inhibits lung fibroblast invasion during transition to injury-induced myofibroblasts. Eur. Respir. J. 63, 2301326 (2024).
Deng, C. C. et al. Single-cell RNA-seq reveals fibroblast heterogeneity and increased mesenchymal fibroblasts in human fibrotic skin diseases. Nat. Commun. 12, 3709 (2021).
Pan, X. et al. Tumour vasculature at single-cell resolution. Nature 632, 429–436 (2024).
Alex, L. et al. Cardiac pericytes acquire a fibrogenic phenotype and contribute to vascular maturation after myocardial infarction. Circulation 148, 882–898 (2023).
Tsukui, T. et al. Collagen-producing lung cell atlas identifies multiple subsets with distinct localization and relevance to fibrosis. Nat. Commun. 11, 1920 (2020).
Ke, D. et al. Macrophage and fibroblast trajectory inference and crosstalk analysis during myocardial infarction using integrated single-cell transcriptomic datasets. J. Transl. Med. 22, 560 (2024).
Abedini, A. et al. Single-cell multi-omic and spatial profiling of human kidneys implicates the fibrotic microenvironment in kidney disease progression. Nat. Genet. 56, 1712–1724 (2024).
Younesi, F. S., Miller, A. E., Barker, T. H., Rossi, F. M. V. & Hinz, B. Fibroblast and myofibroblast activation in normal tissue repair and fibrosis. Nat. Rev. Mol. Cell Biol. 25, 617–638 (2024).
Fang, Y. et al. RUNX2 promotes fibrosis via an alveolar-to-pathological fibroblast transition. Nature 640, 221–230 (2025).
Tsukui, T., Wolters, P. J. & Sheppard, D. Alveolar fibroblast lineage orchestrates lung inflammation and fibrosis. Nature 631, 627–634 (2024).
Mukhatayev, Z., Adilbayeva, A. & Kunz, J. CTHRC1: an emerging hallmark of pathogenic fibroblasts in lung fibrosis. Cells 13, 946 (2024).
Gao, Y. et al. Cross-tissue human fibroblast atlas reveals myofibroblast subtypes with distinct roles in immune modulation. Cancer Cell 42, 1764–1783.e10 (2024).
Lei, L. et al. Portal fibroblasts with mesenchymal stem cell features form a reservoir of proliferative myofibroblasts in liver fibrosis. Hepatology 76, 1360–1375 (2022).
Peisker, F. et al. Mapping the cardiac vascular niche in heart failure. Nat. Commun. 13, 3027 (2022).
Suryawanshi, H. et al. Detection of infiltrating fibroblasts by single-cell transcriptomics in human kidney allografts. PLoS One 17, e0267704 (2022).
Zhong, Y. et al. Single cell RNA-sequencing identifies bone-marrow-derived progenitor cells as a main source of extracellular matrix-producing cells across multiple organ-based fibrotic diseases. Int. J. Biol. Sci. 20, 5027–5042 (2024).
Cadinu, P. et al. Charting the cellular biogeography in colitis reveals fibroblast trajectories and coordinated spatial remodeling. Cell 187, 2010–2028.e30 (2024).
Werner, G. et al. Single-cell transcriptome analysis identifies subclusters with inflammatory fibroblast responses in localized scleroderma. Int. J. Mol. Sci. 24, 9796 (2023).
Lake, B. B. et al. An atlas of healthy and injured cell states and niches in the human kidney. Nature 619, 585–594 (2023).
Meguro, S. et al. Preexisting senescent fibroblasts in the aged bladder create a tumor-permissive niche through CXCL12 secretion. Nat. Aging 4, 1582–1597 (2024).
Hoare, M. & Narita, M. Transmitting senescence to the cell neighbourhood. Nat. Cell Biol. 15, 887–889 (2013).
Schuster, R., Younesi, F., Ezzo, M. & Hinz, B. The role of myofibroblasts in physiological and pathological tissue repair. Cold Spring Harb. Perspect. Biol. 15, a041231 (2023).
Chung, J. J. et al. Single-cell transcriptome profiling of the kidney glomerulus identifies key cell types and reactions to injury. J. Am. Soc. Nephrol. 31, 2341–2354 (2020).
Plikus, M. V. et al. Fibroblasts: origins, definitions, and functions in health and disease. Cell 184, 3852–3872 (2021).
Wohlfahrt, T. et al. PU.1 controls fibroblast polarization and tissue fibrosis. Nature 566, 344–349 (2019).
Chou, Y. H., Pan, S. Y., Shih, H. M. & Lin, S. L. Update of pericytes function and their roles in kidney diseases. J. Formos. Med. Assoc. 123, 307–317 (2024).
Xu, C. et al. Regulation of pericyte metabolic reprogramming restricts the AKI to CKD transition. Metabolism 145, 155592 (2023).
Weiskirchen, R., Weiskirchen, S. & Tacke, F. Organ and tissue fibrosis: molecular signals, cellular mechanisms and translational implications. Mol. Asp. Med. 65, 2–15 (2019).
hAinmhire, E. O. et al. A conditionally immortalized Gli1-positive kidney mesenchymal cell line models myofibroblast transition. Am. J. Physiol. Renal Physiol. 316, F63–F75 (2019).
Chong, S. G., Sato, S., Kolb, M. & Gauldie, J. Fibrocytes and fibroblasts — where are we now. Int. J. Biochem. Cell Biol. 116, 105595 (2019).
Wada, T., Sakai, N., Matsushima, K. & Kaneko, S. Fibrocytes: a new insight into kidney fibrosis. Kidney Int. 72, 269–273 (2007).
Wu, X. et al. CXCL12/CXCR4: an amazing challenge and opportunity in the fight against fibrosis. Ageing Res. Rev. 83, 101809 (2023).
Li, L. et al. Tamibarotene inhibit the accumulation of fibrocyte and alleviate renal fibrosis by IL-17A. Ren. Fail. 42, 1173–1183 (2020).
Kim, J. et al. Circulating and renal fibrocytes are associated with interstitial fibrosis in lupus nephritis. Rheumatology 62, 914–923 (2023).
Schuster, R., Rockel, J. S., Kapoor, M. & Hinz, B. The inflammatory speech of fibroblasts. Immunol. Rev. 302, 126–146 (2021).
Tang, P. M., Nikolic-Paterson, D. J. & Lan, H. Y. Macrophages: versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 15, 144–158 (2019).
Wei, J., Xu, Z. & Yan, X. The role of the macrophage-to-myofibroblast transition in renal fibrosis. Front. Immunol. 13, 934377 (2022).
Kramann, R. et al. Parabiosis and single-cell RNA sequencing reveal a limited contribution of monocytes to myofibroblasts in kidney fibrosis. JCI Insight 3, e99561 (2018).
Kurose, H. Cardiac fibrosis and fibroblasts. Cells 10, 1716 (2021).
Torimoto, K., Elliott, K., Nakayama, Y., Yanagisawa, H. & Eguchi, S. Cardiac and perivascular myofibroblasts, matrifibrocytes, and immune fibrocytes in hypertension; commonalities and differences with myocardial infarction and other cardiovascular diseases. Cardiovasc. Res. 120, 567–580 (2024).
Travers, J. G., Kamal, F. A., Robbins, J., Yutzey, K. E. & Blaxall, B. C. Cardiac fibrosis: the fibroblast awakens. Circ. Res. 118, 1021–1040 (2016).
Moore-Morris, T. et al. Infarct fibroblasts do not derive from bone marrow lineages. Circ. Res. 122, 583–590 (2018).
Chaffin, M. et al. Single-nucleus profiling of human dilated and hypertrophic cardiomyopathy. Nature 608, 174–180 (2022).
Kanisicak, O. et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat. Commun. 7, 12260 (2016).
Tallquist, M. D. Cardiac fibroblast diversity. Annu. Rev. Physiol. 82, 63–78 (2020).
Soliman, H. & Rossi, F. M. V. Cardiac fibroblast diversity in health and disease. Matrix Biol. 91-92, 75–91 (2020).
Kisseleva, T. The origin of fibrogenic myofibroblasts in fibrotic liver. Hepatology 65, 1039–1043 (2017).
Yang, W. et al. Single-cell transcriptomic analysis reveals a hepatic stellate cell-activation roadmap and myofibroblast origin during liver fibrosis in mice. Hepatology 74, 2774–2790 (2021).
Ramachandran, P. et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature 575, 512–518 (2019).
Kim, H. Y. et al. The origin and fate of liver myofibroblasts. Cell Mol. Gastroenterol. Hepatol. 17, 93–106 (2024).
Moss, B. J., Ryter, S. W. & Rosas, I. O. Pathogenic mechanisms underlying idiopathic pulmonary fibrosis. Annu. Rev. Pathol. 17, 515–546 (2022).
Zepp, J. A. et al. Distinct mesenchymal lineages and niches promote epithelial self-renewal and myofibrogenesis in the lung. Cell 170, 1134–1148.e10 (2017).
Ligresti, G. et al. Mesenchymal cells in the lung: evolving concepts and their role in fibrosis. Gene 859, 147142 (2023).
Savin, I. A., Zenkova, M. A. & Sen’kova, A. V. Pulmonary fibrosis as a result of acute lung inflammation: molecular mechanisms, relevant in vivo models, prognostic and therapeutic approaches. Int. J. Mol. Sci. 23, 14959 (2022).
El Agha, E. et al. Two-way conversion between lipogenic and myogenic fibroblastic phenotypes marks the progression and resolution of lung fibrosis. Cell Stem Cell 20, 261–273.e263 (2017).
Adams, T. S. et al. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci. Adv. 6, eaba1983 (2020).
Wang, Y. C. et al. Notch1 promotes the pericyte-myofibroblast transition in idiopathic pulmonary fibrosis through the PDGFR/ROCK1 signal pathway. Exp. Mol. Med. 51, 1–11 (2019).
Yamaguchi, M. et al. Pericyte-myofibroblast transition in the human lung. Biochem. Biophys. Res. Commun. 528, 269–275 (2020).
Ortiz-Zapater, E., Signes-Costa, J., Montero, P. & Roger, I. Lung fibrosis and fibrosis in the lungs: is it all about myofibroblasts? Biomedicines 10, 1423 (2022).
Rock, J. R. et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc. Natl Acad. Sci. USA 108, E1475–E1483 (2011).
Dorrier, C. E. et al. CNS fibroblasts form a fibrotic scar in response to immune cell infiltration. Nat. Neurosci. 24, 234–244 (2021).
Shook, B. A. et al. Myofibroblast proliferation and heterogeneity are supported by macrophages during skin repair. Science 362, eaar2971 (2018).
Wirka, R. C. et al. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single-cell analysis. Nat. Med. 25, 1280–1289 (2019).
He, M., Liu, Z., Li, L. & Liu, Y. Cell-cell communication in kidney fibrosis. Nephrol. Dial. Transpl. 39, 761–769 (2024).
Shi, M. et al. Latent TGF-β structure and activation. Nature 474, 343–349 (2011).
Meng, X. M., Nikolic-Paterson, D. J. & Lan, H. Y. TGF-β: the master regulator of fibrosis. Nat. Rev. Nephrol. 12, 325–338 (2016).
Gu, Y. Y., Liu, X. S., Huang, X. R., Yu, X. Q. & Lan, H. Y. Diverse role of TGF-β in kidney disease. Front. Cell Dev. Biol. 8, 123 (2020).
Ma, T. T. & Meng, X. M. TGF-β/Smad and renal fibrosis. Adv. Exp. Med. Biol. 1165, 347–364 (2019).
Wang, P. et al. Long noncoding RNA lnc-TSI inhibits renal fibrogenesis by negatively regulating the TGF-β/Smad3 pathway. Sci. Transl. Med. 10, eaat2039 (2018).
Finnson, K. W., Almadani, Y. & Philip, A. Non-canonical (non-SMAD2/3) TGF-β signaling in fibrosis: mechanisms and targets. Semin. Cell Dev. Biol. 101, 115–122 (2020).
Kefaloyianni, E. et al. Proximal tubule-derived amphiregulin amplifies and integrates profibrotic EGF receptor signals in kidney fibrosis. J. Am. Soc. Nephrol. 30, 2370–2383 (2019).
Livingston, M. J. et al. Autophagy activates EGR1 via MAPK/ERK to induce FGF2 in renal tubular cells for fibroblast activation and fibrosis during maladaptive kidney repair. Autophagy 20, 1032–1053 (2024).
Ruiz-Ortega, M., Rayego-Mateos, S., Lamas, S., Ortiz, A. & Rodrigues-Diez, R. R. Targeting the progression of chronic kidney disease. Nat. Rev. Nephrol. 16, 269–288 (2020).
Yuan, Q., Tang, B. & Zhang, C. Signaling pathways of chronic kidney diseases, implications for therapeutics. Signal. Transduct. Target. Ther. 7, 182 (2022).
Edeling, M., Ragi, G., Huang, S., Pavenstädt, H. & Susztak, K. Developmental signalling pathways in renal fibrosis: the roles of Notch, Wnt and Hedgehog. Nat. Rev. Nephrol. 12, 426–439 (2016).
Schunk, S. J., Floege, J., Fliser, D. & Speer, T. WNT-β-catenin signalling — a versatile player in kidney injury and repair. Nat. Rev. Nephrol. 17, 172–184 (2021).
Wang, Y., Zhou, C. J. & Liu, Y. Wnt signaling in kidney development and disease. Prog. Mol. Biol. Transl. Sci. 153, 181–207 (2018).
Zhou, S. et al. Cannabinoid receptor type 2 promotes kidney fibrosis through orchestrating β-catenin signaling. Kidney Int. 99, 364–381 (2021).
Xiao, L. et al. Wnt/β-catenin regulates blood pressure and kidney injury in rats. Biochim. Biophys. Acta Mol. Basis Dis. 1865, 1313–1322 (2019).
Liu, Z., Tan, R. J. & Liu, Y. The many faces of matrix metalloproteinase-7 in kidney diseases. Biomolecules 10, 960 (2020).
Zhou, L. et al. Multiple genes of the renin-angiotensin system are novel targets of Wnt/β-catenin signaling. J. Am. Soc. Nephrol. 26, 107–120 (2015).
Miao, J. et al. Wnt/β-catenin/RAS signaling mediates age-related renal fibrosis and is associated with mitochondrial dysfunction. Aging Cell 18, e13004 (2019).
Zuo, Y. & Liu, Y. New insights into the role and mechanism of Wnt/β-catenin signalling in kidney fibrosis. Nephrology 23, 38–43 (2018).
Zhou, D., Tan, R. J. & Liu, Y. Sonic hedgehog signaling in kidney fibrosis: a master communicator. Sci. China Life Sci. 59, 920–929 (2016).
Jiang, J. Hedgehog signaling mechanism and role in cancer. Semin. Cancer Biol. 85, 107–122 (2022).
Guan, Y. et al. Kaempferol inhibits renal fibrosis by suppression of the sonic hedgehog signaling pathway. Phytomedicine 108, 154246 (2023).
Ding, H. et al. Sonic hedgehog signaling mediates epithelial-mesenchymal communication and promotes renal fibrosis. J. Am. Soc. Nephrol. 23, 801–813 (2012).
Zhou, D. et al. Sonic hedgehog is a novel tubule-derived growth factor for interstitial fibroblasts after kidney injury. J. Am. Soc. Nephrol. 25, 2187–2200 (2014).
Gui, Y. et al. Fibroblast expression of transmembrane protein smoothened governs microenvironment characteristics after acute kidney injury. J. Clin. Invest. 134, e165836 (2024).
O’Sullivan, E. D. et al. Indian hedgehog release from TNF-activated renal epithelia drives local and remote organ fibrosis. Sci. Transl. Med. 15, eabn0736 (2023).
Huang, S. et al. Jagged1/Notch2 controls kidney fibrosis via Tfam-mediated metabolic reprogramming. PLoS Biol. 16, e2005233 (2018).
Xiao, X. et al. Hypermethylation leads to the loss of HOXA5, resulting in JAG1 expression and NOTCH signaling contributing to kidney fibrosis. Kidney Int. 106, 98–114 (2024).
AlQudah, M., Hale, T. M. & Czubryt, M. P. Targeting the renin-angiotensin-aldosterone system in fibrosis. Matrix Biol. 91-92, 92–108 (2020).
Zhang, W. et al. Combining experiments and bioinformatics to identify transforming growth factor-β1 as a key regulator in angiotensin II-induced trophoblast senescence. Placenta 152, 31–38 (2024).
Li, L. et al. Oxidatively stressed extracellular microenvironment drives fibroblast activation and kidney fibrosis. Redox Biol. 67, 102868 (2023).
Wang, D., Dai, C., Li, Y. & Liu, Y. Canonical Wnt/β-catenin signaling mediates transforming growth factor-beta1-driven podocyte injury and proteinuria. Kidney Int. 80, 1159–1169 (2011).
Zhou, L., Li, Y., Zhou, D., Tan, R. J. & Liu, Y. Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling. J. Am. Soc. Nephrol. 24, 771–785 (2013).
Zhang, X. et al. Klotho-derived peptide 1 inhibits cellular senescence in the fibrotic kidney by restoring Klotho expression via posttranscriptional regulation. Theranostics 14, 420–435 (2024).
Fu, H. et al. Matrix metalloproteinase-7 protects against acute kidney injury by priming renal tubules for survival and regeneration. Kidney Int. 95, 1167–1180 (2019).
Mo, H. et al. CXCR4 induces podocyte injury and proteinuria by activating β-catenin signaling. Theranostics 12, 767–781 (2022).
Sun, X. et al. Matrix metalloproteinase-10 promotes kidney fibrosis by transactivating β-catenin signaling. Cell Death Discov. 11, 241 (2025).
Long, Y. et al. m6A RNA methylation drives kidney fibrosis by upregulating β-catenin signaling. Int. J. Biol. Sci. 20, 3185–3200 (2024).
Song, D. et al. Insulin-like growth factor 2 mRNA-binding protein 3 promotes kidney injury by regulating β-catenin signaling. JCI Insight 8, e162060 (2023).
Rosenkranz, S. TGF-β1 and angiotensin networking in cardiac remodeling. Cardiovasc. Res. 63, 423–432 (2004).
Liu, Y. Kidney fibrosis: fundamental questions, challenges and perspective. Integr. Med. Nephrol. Androl. 11, e24-00027 (2024).
Tepus, M., Tonoli, E. & Verderio, E. A. M. Molecular profiling of urinary extracellular vesicles in chronic kidney disease and renal fibrosis. Front. Pharmacol. 13, 1041327 (2022).
Zhao, S. et al. Exosomal miR-21 from tubular cells contributes to renal fibrosis by activating fibroblasts via targeting PTEN in obstructed kidneys. Theranostics 11, 8660–8673 (2021).
Chen, S. et al. β-Catenin-controlled tubular cell-derived exosomes play a key role in fibroblast activation via the OPN-CD44 axis. J. Extracell. Vesicles 11, e12203 (2022).
Li, L., Huang, J. & Liu, Y. The extracellular matrix glycoprotein fibrillin-1 in health and disease. Front. Cell Dev. Biol. 11, 1302285 (2023).
Peng, Y. et al. Macrophage promotes fibroblast activation and kidney fibrosis by assembling a vitronectin-enriched microenvironment. Theranostics 13, 3897–3913 (2023).
Li, L., Fu, H. & Liu, Y. The fibrogenic niche in kidney fibrosis: components and mechanisms. Nat. Rev. Nephrol. 18, 545–557 (2022).
Midwood, K. S., Chiquet, M., Tucker, R. P. & Orend, G. Tenascin-C at a glance. J. Cell Sci. 129, 4321–4327 (2016).
Zhu, H. et al. Tenascin-C promotes acute kidney injury to chronic kidney disease progression by impairing tubular integrity via αvβ6 integrin signaling. Kidney Int. 97, 1017–1031 (2020).
Li, L. et al. Fibrillin-1-enriched microenvironment drives endothelial injury and vascular rarefaction in chronic kidney disease. Sci. Adv. 7, eabc7170 (2021).
Zuchtriegel, G. et al. Vitronectin stabilizes intravascular adhesion of neutrophils by coordinating β2 integrin clustering. Haematologica 106, 2641–2653 (2021).
Yamamura, Y. et al. Myocardin-related transcription factor contributes to renal fibrosis through the regulation of extracellular microenvironment surrounding fibroblasts. FASEB J. 37, e23005 (2023).
Chen, Z. et al. Connective tissue growth factor: from molecular understandings to drug discovery. Front. Cell Dev. Biol. 8, 593269 (2020).
Li, Z., Williams, H., Jackson, M. L., Johnson, J. L. & George, S. J. WISP-1 regulates cardiac fibrosis by promoting cardiac fibroblasts’ activation and collagen processing. Cells 13, 989 (2024).
Hinz, B. & Lagares, D. Evasion of apoptosis by myofibroblasts: a hallmark of fibrotic diseases. Nat. Rev. Rheumatol. 16, 11–31 (2020).
Zhao, X. et al. Mechanosensitive Piezo1 channels mediate renal fibrosis. JCI Insight 7, e152330 (2022).
Sun, Z., Costell, M. & Fässler, R. Integrin activation by talin, kindlin and mechanical forces. Nat. Cell Biol. 21, 25–31 (2019).
Tschumperlin, D. J., Ligresti, G., Hilscher, M. B. & Shah, V. H. Mechanosensing and fibrosis. J. Clin. Invest. 128, 74–84 (2018).
Gupta, V., Gupta, I., Park, J., Bram, Y. & Schwartz, R. E. Hedgehog signaling demarcates a niche of fibrogenic peribiliary mesenchymal cells. Gastroenterology 159, 624–638 (2020).
Gonzalez-Sanchez, E. et al. The hepatocyte epidermal growth factor receptor (EGFR) pathway regulates the cellular interactome within the liver fibrotic niche. J. Pathol. 263, 482–495 (2024).
Königshoff, M. & Eickelberg, O. Listen to the WNT; it talks: WNT7A drives epithelial-mesenchymal cross-talk within the fibrotic niche in idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 68, 239–240 (2023).
Lausecker, F., Lennon, R. & Randles, M. J. The kidney matrisome in health, aging, and disease. Kidney Int. 102, 1000–1012 (2022).
Naba, A. Ten years of extracellular matrix proteomics: accomplishments, challenges, and future perspectives. Mol. Cell Proteom. 22, 100528 (2023).
Herrera, J., Henke, C. A. & Bitterman, P. B. Extracellular matrix as a driver of progressive fibrosis. J. Clin. Invest. 128, 45–53 (2018).
Mathot, L. & Stenninger, J. Behavior of seeds and soil in the mechanism of metastasis: a deeper understanding. Cancer Sci. 103, 626–631 (2012).
Li, L. et al. Proteomic landscape of the extracellular matrix in the fibrotic kidney. Kidney Int. 103, 1063–1076 (2023).
Barker, H. E., Bird, D., Lang, G. & Erler, J. T. Tumor-secreted LOXL2 activates fibroblasts through FAK signaling. Mol. Cancer Res. 11, 1425–1436 (2013).
Tian, S. et al. SERPINH1 regulates EMT and gastric cancer metastasis via the Wnt/β-catenin signaling pathway. Aging 12, 3574–3593 (2020).
Vlad, M. L. et al. Therapeutic S100A8/A9 inhibition reduces NADPH oxidase expression, reactive oxygen species production and NLRP3 inflammasome priming in the ischemic myocardium. Eur. J. Pharmacol. 996, 177575 (2025).
Stasi, A. et al. Emerging role of lipopolysaccharide binding protein in sepsis-induced acute kidney injury. Nephrol. Dial. Transpl. 32, 24–31 (2017).
Chen, S. et al. Tenascin-C protects against acute kidney injury by recruiting Wnt ligands. Kidney Int. 95, 62–74 (2019).
Guo, C. et al. Crosstalk between proximal tubular epithelial cells and other interstitial cells in tubulointerstitial fibrosis after renal injury. Front. Endocrinol. 14, 1256375 (2023).
Lan, R. et al. Mitochondrial pathology and glycolytic shift during proximal tubule atrophy after ischemic AKI. J. Am. Soc. Nephrol. 27, 3356–3367 (2016).
Zhou, D. et al. Fibroblast-specific β-Catenin signaling dictates the outcome of AKI. J. Am. Soc. Nephrol. 29, 1257–1271 (2018).
Nakamura, J. et al. Myofibroblasts acquire retinoic acid-producing ability during fibroblast-to-myofibroblast transition following kidney injury. Kidney Int. 95, 526–539 (2019).
Zhang, Y. et al. Advances in understanding the effects of erythropoietin on renal fibrosis. Front. Med. 7, 47 (2020).
Pakshir, P. & Hinz, B. The big five in fibrosis: macrophages, myofibroblasts, matrix, mechanics, and miscommunication. Matrix Biol. 68-69, 81–93 (2018).
Ramanujam, D. et al. MicroRNA-21-dependent macrophage-to-fibroblast signaling determines the cardiac response to pressure overload. Circulation 143, 1513–1525 (2021).
Venter, C., Myburgh, K. H. & Niesler, C. U. Co-culture of pro-inflammatory macrophages and myofibroblasts: evaluating morphological phenotypes and screening the effects of signaling pathway inhibitors. Physiol. Rep. 9, e14704 (2021).
Yang, F. et al. Crosstalk between hepatic stellate cells and surrounding cells in hepatic fibrosis. Int. Immunopharmacol. 99, 108051 (2021).
Cai, X. et al. Intercellular crosstalk of hepatic stellate cells in liver fibrosis: new insights into therapy. Pharmacol. Res. 155, 104720 (2020).
McDaniels, J. M. et al. Single nuclei transcriptomics delineates complex immune and kidney cell interactions contributing to kidney allograft fibrosis. Kidney Int. 103, 1077–1092 (2023).
Wei, K., Nguyen, H. N. & Brenner, M. B. Fibroblast pathology in inflammatory diseases. J. Clin. Invest. 131, e149538 (2021).
Chen, X., Wu, Y., Jia, S. & Zhao, M. Fibroblast: a novel target for autoimmune and inflammatory skin diseases therapeutics. Clin. Rev. Allergy Immunol. 66, 274–293 (2024).
Korsunsky, I. et al. Cross-tissue, single-cell stromal atlas identifies shared pathological fibroblast phenotypes in four chronic inflammatory diseases. Med 3, 481–518.e414 (2022).
Davidson, S. et al. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat. Rev. Immunol. 21, 704–717 (2021).
Sinha, S. et al. Fibroblast inflammatory priming determines regenerative versus fibrotic skin repair in reindeer. Cell 185, 4717–4736.e4725 (2022).
Wu, L. et al. Crosstalk between myofibroblasts and macrophages: a regulative factor of valvular fibrosis in calcific aortic valve disease. Cell Biol. Int. 47, 754–767 (2023).
Pakshir, P. et al. Dynamic fibroblast contractions attract remote macrophages in fibrillar collagen matrix. Nat. Commun. 10, 1850 (2019).
Mincham, K. T., Bruno, N., Singanayagam, A. & Snelgrove, R. J. Our evolving view of neutrophils in defining the pathology of chronic lung disease. Immunology 164, 701–721 (2021).
Cao, C., Yao, Y. & Zeng, R. Lymphocytes: versatile participants in acute kidney injury and progression to chronic kidney disease. Front. Physiol. 12, 729084 (2021).
Zhang, M., Chen, H., Qian, H. & Wang, C. Characterization of the skin keloid microenvironment. Cell Commun. Signal. 21, 207 (2023).
Gewin, L., Zent, R. & Pozzi, A. Progression of chronic kidney disease: too much cellular talk causes damage. Kidney Int. 91, 552–560 (2017).
Tanaka, S., Portilla, D. & Okusa, M. D. Role of perivascular cells in kidney homeostasis, inflammation, repair and fibrosis. Nat. Rev. Nephrol. 19, 721–732 (2023).
Shim, J. et al. Integrated analysis of single-cell and spatial transcriptomics in keloids: highlights on fibrovascular interactions in keloid pathogenesis. J. Invest. Dermatol. 142, 2128–2139.e11 (2022).
Yazdani, S., Bansal, R. & Prakash, J. Drug targeting to myofibroblasts: implications for fibrosis and cancer. Adv. Drug. Deliv. Rev. 121, 101–116 (2017).
Chang, F. C. et al. Angiopoietin-2 inhibition attenuates kidney fibrosis by hindering chemokine C-C motif ligand 2 expression and apoptosis of endothelial cells. Kidney Int. 102, 780–797 (2022).
Huang, Z. et al. Key role for EphB2 receptor in kidney fibrosis. Clin. Sci. 135, 2127–2142 (2021).
Jun, J. I. & Lau, L. F. Resolution of organ fibrosis. J. Clin. Invest. 128, 97–107 (2018).
Kuehl, T. & Lagares, D. BH3 mimetics as anti-fibrotic therapy: unleashing the mitochondrial pathway of apoptosis in myofibroblasts. Matrix Biol. 68-69, 94–105 (2018).
Lagares, D. et al. Targeted apoptosis of myofibroblasts with the BH3 mimetic ABT-263 reverses established fibrosis. Sci. Transl. Med. 9, eaal3765 (2017).
Liu, X. et al. Kidney tubular epithelial cells control interstitial fibroblast fate by releasing TNFAIP8-encapsulated exosomes. Cell Death Dis. 14, 672 (2023).
Krizhanovsky, V. et al. Senescence of activated stellate cells limits liver fibrosis. Cell 134, 657–667 (2008).
Gorgoulis, V. et al. Cellular senescence: defining a path forward. Cell 179, 813–827 (2019).
Merkt, W., Zhou, Y., Han, H. & Lagares, D. Myofibroblast fate plasticity in tissue repair and fibrosis: deactivation, apoptosis, senescence and reprogramming. Wound Repair. Regen. 29, 678–691 (2021).
Rangarajan, S. et al. Mitochondrial uncoupling protein-2 reprograms metabolism to induce oxidative stress and myofibroblast senescence in age-associated lung fibrosis. Aging Cell 21, e13674 (2022).
Kato, K. et al. Impaired myofibroblast dedifferentiation contributes to nonresolving fibrosis in aging. Am. J. Respir. Cell Mol. Biol. 62, 633–644 (2020).
Caligiuri, A., Gentilini, A., Pastore, M., Gitto, S. & Marra, F. Cellular and molecular mechanisms underlying liver fibrosis regression. Cells 10, 2759 (2021).
Aguado, B. A. et al. Transcatheter aortic valve replacements alter circulating serum factors to mediate myofibroblast deactivation. Sci. Transl. Med. 11, eaav3233 (2019).
Plikus, M. V. et al. Regeneration of fat cells from myofibroblasts during wound healing. Science 355, 748–752 (2017).
Tani, H. et al. Direct reprogramming improves cardiac function and reverses fibrosis in chronic myocardial infarction. Circulation 147, 223–238 (2023).
Wu, J. et al. Improved factor combination for in vivo reprogramming of cardiac myofibroblast to cardiomyocyte-like cell with dual recombinase tracing. Circulation 148, 1728–1731 (2023).
Song, G. et al. Direct reprogramming of hepatic myofibroblasts into hepatocytes in vivo attenuates liver fibrosis. Cell Stem Cell 18, 797–808 (2016).
Raghu, G. et al. Pamrevlumab for idiopathic pulmonary fibrosis: the ZEPHYRUS-1 randomized clinical trial. JAMA 332, 380–389 (2024).
Edmonston, D., Grabner, A. & Wolf, M. FGF23 and klotho at the intersection of kidney and cardiovascular disease. Nat. Rev. Cardiol. 21, 11–24 (2024).
Zhou, L. et al. Klotho ameliorates kidney injury and fibrosis and normalizes blood pressure by targeting the renin-angiotensin system. Am. J. Pathol. 185, 3211–3223 (2015).
Wang, Y. & Zhao, J. The protective function of ɑKlotho in chronic kidney disease: evidence and therapeutic implications. Integ Med. Nephrol. Androl. 11, e24-00021 (2024).
Li, H. et al. Nanoparticle-mediated Klotho gene therapy prevents acute kidney injury to chronic kidney disease transition through regulating PPARα signaling in renal tubular epithelial cells. Biomaterials 315, 122926 (2025).
Yuan, Q. et al. A Klotho-derived peptide protects against kidney fibrosis by targeting TGF-β signaling. Nat. Commun. 13, 438 (2022).
Chen, X. et al. Klotho-derived peptide 6 ameliorates diabetic kidney disease by targeting Wnt/β-catenin signaling. Kidney Int. 102, 506–520 (2022).
Zhao, M. et al. Targeting fibrosis, mechanisms and clinical trials. Signal. Transduct. Target. Ther. 7, 206 (2022).
Hao, S. et al. Targeted inhibition of β-catenin/CBP signaling ameliorates renal interstitial fibrosis. J. Am. Soc. Nephrol. 22, 1642–1653 (2011).
Park, J. S. et al. Targeting of dermal myofibroblasts through death receptor 5 arrests fibrosis in mouse models of scleroderma. Nat. Commun. 10, 1128 (2019).
Tan, R. J. & Liu, Y. Matrix metalloproteinases in kidney homeostasis and diseases. Am. J. Physiol. Renal Physiol. 302, F1351–F1361 (2012).
Zuo, Y. et al. Identification of matrix metalloproteinase-10 as a key mediator of podocyte injury and proteinuria. Kidney Int. 100, 837–849 (2021).
Qin, L. et al. Mesenchymal stem cells in fibrotic diseases-the two sides of the same coin. Acta Pharmacol. Sin. 44, 268–287 (2023).
Nazarie Ignat, S. R., Gharbia, S., Hermenean, A., Dinescu, S. & Costache, M. Regenerative potential of mesenchymal stem cells’ (MSCs) secretome for liver fibrosis therapies. Int. J. Mol. Sci. 22, 13292 (2021).
Glassberg, M. K. et al. Allogeneic human mesenchymal stem cells in patients with idiopathic pulmonary fibrosis via intravenous delivery (AETHER): a phase I safety clinical trial. Chest 151, 971–981 (2017).
Han, M. M. et al. Nanoengineered mesenchymal stem cell therapy for pulmonary fibrosis in young and aged mice. Sci. Adv. 9, eadg5358 (2023).
Chen, L. et al. Exosomes derived from GDNF-modified human adipose mesenchymal stem cells ameliorate peritubular capillary loss in tubulointerstitial fibrosis by activating the SIRT1/eNOS signaling pathway. Theranostics 10, 9425–9442 (2020).
Huang, Y. & Yang, L. Mesenchymal stem cell-derived extracellular vesicles in therapy against fibrotic diseases. Stem Cell Res. Ther. 12, 435 (2021).
Ume, A. C. et al. Tacrolimus induces fibroblast-to-myofibroblast transition via a TGF-β-dependent mechanism to contribute to renal fibrosis. Am. J. Physiol. Renal Physiol. 324, F433–F445 (2023).
Qiang, P. et al. Esaxerenone inhibits the macrophage-to-myofibroblast transition through mineralocorticoid receptor/TGF-β1 pathway in mice induced with aldosterone. Front. Immunol. 13, 948658 (2022).
Cao, Y. et al. Integrin β8 prevents pericyte-myofibroblast transition and renal fibrosis through inhibiting the TGF-β1/TGFBR1/Smad3 pathway in diabetic kidney disease. Transl. Res. 265, 36–50 (2024).
Jacobs, M. E., de Vries, D. K., Engelse, M. A., Dumas, S. J. & Rabelink, T. J. Endothelial to mesenchymal transition in kidney fibrosis. Nephrol. Dial. Transpl. 39, 752–760 (2024).
Livingston, M. J. et al. Tubular cells produce FGF2 via autophagy after acute kidney injury leading to fibroblast activation and renal fibrosis. Autophagy 19, 256–277 (2023).
Dolivo, D. M. Anti-fibrotic effects of pharmacologic FGF-2: a review of recent literature. J. Mol. Med. 100, 847–860 (2022).
Ai, J. Y., Liu, C. F., Zhang, W. & Rao, G. W. Current status of drugs targeting PDGF/PDGFR. Drug. Discov. Today 29, 103989 (2024).
Steele, H. et al. TNF superfamily control of tissue remodeling and fibrosis. Front. Immunol. 14, 1219907 (2023).
Evdokiou, A. et al. Characterization of burn eschar pericytes. J. Clin. Med. 9, 606 (2020).
Li, H. et al. A recombinant IL-1β vaccine attenuates bleomycin-induced pulmonary fibrosis in mice. Vaccine 42, 3774–3788 (2024).
Zhang, W. J., Chen, S. J., Zhou, S. C., Wu, S. Z. & Wang, H. Inflammasomes and fibrosis. Front. Immunol. 12, 643149 (2021).
Doke, T. et al. Single-cell analysis identifies the interaction of altered renal tubules with basophils orchestrating kidney fibrosis. Nat. Immunol. 23, 947–959 (2022).
Chen, Y., Zhou, J., Xu, S. & Nie, J. Role of interleukin-6 family cytokines in organ fibrosis. Kidney Dis. 9, 239–253 (2023).
Takagaki, Y. et al. Endothelial autophagy deficiency induces IL6-dependent endothelial mesenchymal transition and organ fibrosis. Autophagy 16, 1905–1914 (2020).
He, S., Yao, L. & Li, J. Role of MCP-1/CCR2 axis in renal fibrosis: mechanisms and therapeutic targeting. Medicine 102, e35613 (2023).
Chen, Y., Zou, H., Lu, H., Xiang, H. & Chen, S. Research progress of endothelial-mesenchymal transition in diabetic kidney disease. J. Cell Mol. Med. 26, 3313–3322 (2022).
He, X. et al. Research progress of pericytes in pulmonary fibrosis. Front. Biosci. 29, 141 (2024).
Zhou, D. et al. Non-canonical Wnt/calcium signaling is protective against podocyte injury and glomerulosclerosis. Kidney Int. 102, 96–107 (2022).
Luo, Y. et al. Inhibitory effects of rhein on renal interstitial fibrosis via the SHH-Gli1 signal pathway. Evid. Based Complement. Altern. Med. 2022, 4398265 (2022).
Hong, W. et al. Epithelial and interstitial Notch1 activity contributes to the myofibroblastic phenotype and fibrosis. Cell Commun. Signal. 17, 145 (2019).
Yuan, C., Ni, L., Zhang, C. & Wu, X. The role of Notch3 signaling in kidney disease. Oxid. Med. Cell Longev. 2020, 1809408 (2020).
Panizo, S. et al. Fibrosis in chronic kidney disease: pathogenesis and consequences. Int. J. Mol. Sci. 22, 408 (2021).
Zhuang, T. et al. ALKBH5-mediated m6A modification of IL-11 drives macrophage-to-myofibroblast transition and pathological cardiac fibrosis in mice. Nat. Commun. 15, 1995 (2024).
Feng, X., Su, H., He, X., Chen, J. X. & Zeng, H. SIRT3 deficiency sensitizes angiotensin-II-induced renal fibrosis. Cells 9, 2510 (2020).
Salminen, A. The role of immunosuppressive myofibroblasts in the aging process and age-related diseases. J. Mol. Med. 101, 1169–1189 (2023).
Zhang, L. et al. Knockout RAGE alleviates cardiac fibrosis through repressing endothelial-to-mesenchymal transition (EndMT) mediated by autophagy. Cell Death Dis. 12, 470 (2021).
Liu, J. et al. RAGE pathways play an important role in regulation of organ fibrosis. Life Sci. 323, 121713 (2023).
Acknowledgements
We thank Dr. Li Li and other members of the Liu laboratory for many stimulating discussions. The author’s work was supported by the National Natural Science Foundation of China (NSFC) grants 82230020 and 82430026, the Key Technologies R&D Program of Guangdong Province (2023B1111030004), the Postdoctoral Fellowship Program of CPSF (GZB20240301) and funds from the Guangdong Provincial Clinical Research Center for Kidney Disease, Guangdong Key Laboratory of Organ Failure Research and Guangdong Provincial Institute of Nephrology.
Author information
Authors and Affiliations
Contributions
Y.L. conceived the article and provided the outlines of the manuscript. X.Z. and Y.Z. researched literature for the article, wrote the manuscript and created the figures. Y.L. wrote and revised the manuscript. All authors made substantial contributions to discussions of the content and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks Rafael Kramann, David Lagares and Shougang Zhuang for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, X., Zhang, Y. & Liu, Y. Fibroblast activation and heterogeneity in fibrotic disease. Nat Rev Nephrol 21, 613–632 (2025). https://doi.org/10.1038/s41581-025-00969-8
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41581-025-00969-8
This article is cited by
-
Lung-targeted RNA delivery systems: strategies and therapeutic applications
Journal of Nanobiotechnology (2026)
-
Neutrophils as critical orchestrators of chronic inflammation
Cellular & Molecular Immunology (2026)