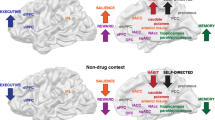
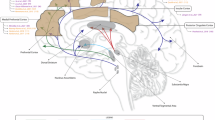
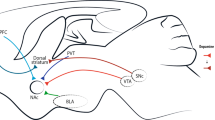
Abstract
Chronic relapse is a hallmark of substance-use disorders (SUDs), but many people with SUDs do recover and eventually enter remission. Many preclinical studies in this field aim to identify interventions that can precipitate recovery by reversing or erasing the neuronal circuit changes caused by chronic drug use. A better understanding of remission from SUDs can also come from preclinical studies that model factors known to influence recovery in humans, such as the negative consequences of drug use and positive environmental influences. In this Perspective we discuss human neuroimaging studies that have provided information about recovery from SUDs and highlight mechanisms identified in preclinical studies — such as the reconfiguration of neuronal circuits — that could contribute to remission. We also analyse how studies of memory and forgetting can provide insights into the mechanisms of remission. Overall, we propose that remission can be driven by the introduction of new neuronal changes (which outcompete those induced by drugs) as well as by the erasure of drug-induced changes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
O’Brien, C. P. & McLellan, A. T. Myths about the treatment of addiction. Lancet 347, 237–240 (1996).
Brecht, M. L. & Herbeck, D. Time to relapse following treatment for methamphetamine use: a long-term perspective on patterns and predictors. Drug. Alcohol Depend. 139, 18–25 (2014).
McLellan, A. T., Lewis, D. C., O’Brien, C. P. & Kleber, H. D. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. J. Am. Med. Assoc. 284, 1689–1695 (2000).
Burman, S. The challenge of sobriety: natural recovery without treatment and self-help groups. J. Subst. Abus. 9, 41–61 (1997).
Kelly, J. F., Greene, M. C., Bergman, B. G., White, W. L. & Hoeppner, B. B. How many recovery attempts does it take to successfully resolve an alcohol or drug problem? Estimates and correlates from a National Study of Recovering U.S. adults. Alcohol. Clin. Exp. Res. 43, 1533–1544 (2019).
SAMHSA’s working definition of recovery. SAMHSA https://store.samhsa.gov/sites/default/files/pep12-recdef.pdf (2012).
About Recovery. NIDA https://nida.nih.gov/research-topics/recovery#:~:Text=Recovery%20is%20a%20process%20of,This%20is%20called%20remission (2019).
International Classification of Diseases 11th Revision (ICD-11). World Health Organization https://www.who.int/standards/classifications/classification-of-diseases (2021).
Robins, L. N. & Regier, D. A. (eds) Psychiatric Disorders In America: The Epidemiologic Catchment Area Study (Maxwell Macmillan International, 1991).
Heyman, G. M. Quitting drugs: quantitative and qualitative features. Annu. Rev. Clin. Psychol. 9, 29–59 (2013).
Kessler, R. C. et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 593–602 (2005).
Kessler, R. C. et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. results from the National Comorbidity Survey. Arch. Gen. Psychiatry 51, 8–19 (1994).
Stinson, F. S. et al. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug. Alcohol Depend. 80, 105–116 (2005).
Jones, C. M., Noonan, R. K. & Compton, W. M. Prevalence and correlates of ever having a substance use problem and substance use recovery status among adults in the United States, 2018. Drug. Alcohol Depend. 214, 108169 (2020).
Fan, A. Z., Chou, S. P., Zhang, H., Jung, J. & Grant, B. F. Prevalence and correlates of past-year recovery from DSM-5 alcohol use disorder: results from National Epidemiologic Survey On Alcohol And Related Conditions III. Alcohol. Clin. Exp. Res. 43, 2406–2420 (2019).
Key substance use and mental health indicators in the United States: results from the 2021 National Survey on Drug Use and Health. SAMHSA 64–72 https://www.samhsa.gov/data/sites/default/files/reports/rpt39443/2021NSDUHFFRRev010323.pdf (2022).
Lopez-Quintero, C. et al. Probability and predictors of remission from life-time nicotine, alcohol, cannabis or cocaine dependence: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Addiction 106, 657–669 (2011).
Day, E., Manitsa, I., Farley, A. & Kelly, J. F. The UK National Recovery Survey: nationally representative survey of people overcoming a drug or alcohol problem. BJPsych Open 10, e67 (2024).
Pongsavee, K., Payakkakom, A., Phukao, D. & Guadamuz, T. E. Natural recovery from alcohol: a systematic review of the literature 2006–2019. J. Subst. Use 28, 166–171 (2023).
Moos, R. H. & Finney, J. W. Commentary on Lopez-Quintero et al. (2011): remission and relapse — the yin–yang of addictive disorders. Addiction 106, 670–671 (2011).
Witkiewitz, K., Pfund, R. A. & Tucker, J. A. Mechanisms of behavior change in substance use disorder with and without formal treatment. Annu. Rev. Clin. Psychol. 18, 497–525 (2022).
Klingemann, H., Sobell, M. B. & Sobell, L. C. Continuities and changes in self-change research. Addiction 105, 1510–1518 (2010).
Winick, C. S. Maturing out of narcotic addiction. Bull. Narc. 14, 1–7 (1962).
Stall, R. & Biernacki, P. Spontaneous remission from the problematic use of substances: an inductive model derived from a comparative analysis of the alcohol, opiate, tobacco, and food/obesity literatures. Int. J. Addict. 21, 1–23 (1986).
Sobell, L. C., Sobell, M. B., Toneatto, T. & Leo, G. I. What triggers the resolution of alcohol problems without treatment. Alcohol. Clin. Exp. Res. 17, 217–224, (1993).
Waldorf, D. Natural recovery from addiction: some social-psychological processes of untreated recovery. J. Drug. Issues 13, 237–280 (1983).
Heilig, M. et al. Addiction as a brain disease revised: why it still matters, and the need for consilience. Neuropsychopharmacology 46, 1715–1723 (2021).
Lamb, R. J., Stark, H. G. & Ginsburg, B. C. Implications of there being many paths to addiction and recovery. Pharmacol. Biochem. Behav. 211, 173299 (2021).
Godino, A. et al. Transcriptional control of nucleus accumbens neuronal excitability by retinoid X receptor alpha tunes sensitivity to drug rewards. Neuron 111, 1453–1467 e1457 (2023).
Kennedy, P. J. et al. Class I HDAC inhibition blocks cocaine-induced plasticity by targeted changes in histone methylation. Nat. Neurosci. 16, 434–440 (2013).
Li, Y. et al. Synaptic mechanism underlying serotonin modulation of transition to cocaine addiction. Science 373, 1252–1256 (2021).
Pascoli, V. et al. Stochastic synaptic plasticity underlying compulsion in a model of addiction. Nature 564, 366–371 (2018).
Nestler, E. J. & Luscher, C. The molecular basis of drug addiction: linking epigenetic to synaptic and circuit mechanisms. Neuron 102, 48–59 (2019).
Bordeaux, P. & Koob, G. F. Escaping Addiction: Resetting the Brain for Success (Rowman & Littlefield, 2023).
Balodis, I. M. et al. Neurofunctional reward processing changes in cocaine dependence during recovery. Neuropsychopharmacology 41, 2112–2121 (2016).
Yip, S. W., Scheinost, D., Potenza, M. N. & Carroll, K. M. Connectome-based prediction of cocaine abstinence. Am. J. Psychiatry 176, 156–164 (2019).
Parvaz, M. A., Rabin, R. A., Adams, F. & Goldstein, R. Z. Structural and functional brain recovery in individuals with substance use disorders during abstinence: a review of longitudinal neuroimaging studies. Drug. Alcohol Depend. 232, 109319 (2022).
Goldstein, R. Z. & Volkow, N. D. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 12, 652–669, (2011).
Maggioni, E. et al. Brain volumes in alcohol use disorder: do females and males differ? A whole-brain magnetic resonance imaging mega-analysis. Hum. Brain Mapp. 44, 4652–4666 (2023).
Spindler, C., Mallien, L., Trautmann, S., Alexander, N. & Muehlhan, M. A coordinate-based meta-analysis of white matter alterations in patients with alcohol use disorder. Transl. Psychiatry 12, 40 (2022).
Yang, X. et al. Cortical and subcortical gray matter shrinkage in alcohol-use disorders: a voxel-based meta-analysis. Neurosci. Biobehav. Rev. 66, 92–103 (2016).
Garavan, H., Brennan, K. L., Hester, R. & Whelan, R. The neurobiology of successful abstinence. Curr. Opin. Neurobiol. 23, 668–674 (2013).
Hammond, C. J., Allick, A., Rahman, N. & Nanavati, J. Structural and functional neural targets of addiction treatment in adolescents and young adults: a systematic review and meta-analysis. J. Child. Adolesc. Psychopharmacol. 29, 498–507 (2019).
Costumero, V. et al. Distance disintegration characterizes node-level topological dysfunctions in cocaine addiction. Addict. Biol. 26, e13072 (2021).
Zilverstand, A. et al. Whole-brain resting-state connectivity underlying impaired inhibitory control during early versus longer-term abstinence in cocaine addiction. Mol. Psychiatry 28, 3355–3364 (2023).
Huang, Y. et al. Association of cortico-striatal engagement during cue reactivity, reappraisal, and savoring of drug and non-drug stimuli with craving in heroin addiction. Am. J. Psychiatry 181, 153–165 (2024).
Joutsa, J. et al. Brain lesions disrupting addiction map to a common human brain circuit. Nat. Med. 28, 1249–1255 (2022).
Fox, M. D. Mapping symptoms to brain networks with the human connectome. N. Engl. J. Med. 379, 2237–2245 (2018).
Venniro, M., Banks, M. L., Heilig, M., Epstein, D. H. & Shaham, Y. Improving translation of animal models of addiction and relapse by reverse translation. Nat. Rev. Neurosci. 21, 625–643 (2020).
Humphreys, K. & Bickel, W. K. Toward a neuroscience of long-term recovery from addiction. JAMA Psychiatry 75, 875–876 (2018).
Ceceli, A. O., Bradberry, C. W. & Goldstein, R. Z. The neurobiology of drug addiction: cross-species insights into the dysfunction and recovery of the prefrontal cortex. Neuropsychopharmacology 47, 276–291 (2022).
Bischof, G., Rumpf, H. J., Hapke, U., Meyer, C. & John, U. Factors influencing remission from alcohol dependence without formal help in a representative population sample. Addiction 96, 1327–1336 (2001).
Scherbaum, N. & Specka, M. Factors influencing the course of opiate addiction. Int. J. Meth. Psychiatric Res. 17, S39–S44 (2008).
Granfield, R. & Cloud, W. Social context and “natural recovery”: the role of social capital in the resolution of drug-associated problems. Subst. Use Misuse 36, 1543–1570 (2001).
Acuff, S. F., MacKillop, J. & Murphy, J. G. A contextualized reinforcer pathology approach to addiction. Nat. Rev. Psychol. 2, 309–323 (2023).
Pickard, H. & Ahmed, S. H. in Addiction and Choice: Rethinking the Relationship 29–48 (Oxford Univ. Press, 2017).
Engeln, M. & Ahmed, S. H. The multiple faces of footshock punishment in animal research on addiction. Neurobiol. Learn. Mem. 213, 107955 (2024).
Kim, C. K. et al. Molecular and circuit-dynamical identification of top-down neural mechanisms for restraint of reward seeking. Cell 170, 1013–1027.e1014 (2017).
Park, J. & Moghaddam, B. Risk of punishment influences discrete and coordinated encoding of reward-guided actions by prefrontal cortex and VTA neurons. eLife 6, e30056 (2017).
Bellone, C., Loureiro, M. & Luscher, C. Drug-evoked synaptic plasticity of excitatory transmission in the ventral tegmental area. Cold Spring Harb. Perspect. Med. 11, a039701 (2021).
Lammel, S., Ion, D. I., Roeper, J. & Malenka, R. C. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron 70, 855–862 (2011).
de Jong, J. W. et al. A neural circuit mechanism for encoding aversive stimuli in the mesolimbic dopamine system. Neuron 101, 133–151.e137 (2019).
Zhou, K. et al. Reward and aversion processing by input-defined parallel nucleus accumbens circuits in mice. Nat. Commun. 13, 6244 (2022).
Vollmer, K. M. et al. An opioid-gated thalamoaccumbal circuit for the suppression of reward seeking in mice. Nat. Commun. 13, 6865 (2022).
Choi, E. A., Jean-Richard-Dit-Bressel, P., Clifford, C. W. G. & McNally, G. P. Paraventricular thalamus controls behavior during motivational conflict. J. Neurosci. 39, 4945–4958 (2019).
Choi, E. A. & McNally, G. P. Paraventricular thalamus balances danger and reward. J. Neurosci. 37, 3018–3029 (2017).
King, S. G. et al. Prefrontal-habenular microstructural impairments in human cocaine and heroin addiction. Neuron 110, 3820–3832.e3824 (2022).
Mathis, V. & Kenny, P. J. From controlled to compulsive drug-taking: the role of the habenula in addiction. Neurosci. Biobehav. Rev. 106, 102–111 (2019).
Meye, F. J. et al. Shifted pallidal co-release of GABA and glutamate in habenula drives cocaine withdrawal and relapse. Nat. Neurosci. 19, 1019–1024 (2016).
Ables, J. L., Park, K. & Ibanez-Tallon, I. Understanding the habenula: a major node in circuits regulating emotion and motivation. Pharmacol. Res. 190, 106734 (2023).
Goldstein, R. Z. et al. Anterior cingulate cortex hypoactivations to an emotionally salient task in cocaine addiction. Proc. Natl Acad. Sci. USA 106, 9453–9458 (2009).
Goldstein, R. Z. et al. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? Am. J. Psychiatry 164, 43–51 (2007).
Jean-Richard-Dit-Bressel, P., Killcross, S. & McNally, G. P. Behavioral and neurobiological mechanisms of punishment: implications for psychiatric disorders. Neuropsychopharmacology 43, 1639–1650 (2018).
Ma, C. et al. Medial orbitofrontal cortex regulates instrumental conditioned punishment, but not pavlovian conditioned fear. Cereb. Cortex Commun. 1, tgaa039 (2020).
Orsini, C. A., Trotta, R. T., Bizon, J. L. & Setlow, B. Dissociable roles for the basolateral amygdala and orbitofrontal cortex in decision-making under risk of punishment. J. Neurosci. 35, 1368–1379 (2015).
Cadet, J. L., Patel, R. & Jayanthi, S. Compulsive methamphetamine taking and abstinence in the presence of adverse consequences: epigenetic and transcriptional consequences in the rat brain. Pharmacol. Biochem. Behav. 179, 98–108 (2019).
Torres, O. V., Jayanthi, S., McCoy, M. T. & Cadet, J. L. Selective activation of striatal NGF-TrkA/p75NTR/MAPK intracellular signaling in rats that show suppression of methamphetamine intake 30 days following drug abstinence. Int. J. Neuropsychopharmacol. 21, 281–290 (2018).
Swanson, A. M., DePoy, L. M. & Gourley, S. L. Inhibiting Rho kinase promotes goal-directed decision making and blocks habitual responding for cocaine. Nat. Commun. 8, 1861 (2017).
Blackwood, C. A., McCoy, M. T., Ladenheim, B. & Cadet, J. L. Escalated oxycodone self-administration and punishment: differential expression of opioid receptors and immediate early genes in the rat dorsal striatum and prefrontal cortex. Front. Neurosci. 13, 1392 (2019).
McClung, C. A. et al. DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res. Mol. Brain Res. 132, 146–154 (2004).
Nestler, E. J. FosB: a transcriptional regulator of stress and antidepressant responses. Eur. J. Pharmacol. 753, 66–72 (2015).
Renthal, W. et al. Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins. Neuron 62, 335–348 (2009).
Pitchers, K. K. et al. Natural and drug rewards act on common neural plasticity mechanisms with DeltaFosB as a key mediator. J. Neurosci. 33, 3434–3442 (2013).
Cadet, J. L. et al. Genome-wide DNA hydroxymethylation identifies potassium channels in the nucleus accumbens as discriminators of methamphetamine addiction and abstinence. Mol. Psychiatry 22, 1196–1204 (2017).
Pascoli, V. et al. Cell-type specific synaptic plasticity in dorsal striatum is associated with punishment-resistance compulsive-like cocaine self-administration in mice. Neuropsychopharmacology 48, 448–458 (2023).
Krasnova, I. N. et al. Incubation of methamphetamine and palatable food craving after punishment-induced abstinence. Neuropsychopharmacology 39, 2008–2016 (2014).
Negishi, K., Fredriksson, I., Bossert, J. M., Zangen, A. & Shaham, Y. Relapse after electric barrier-induced voluntary abstinence: a review. Curr. Opin. Neurobiol. 86, 102856 (2024).
Hill, K., Bodurtha, P. J., Winkelman, T. N. A. & Howell, B. A. Postrelease risk of overdose and all-cause death among persons released from jail or prison: minnesota, March 2020–December 2021. Am. J. Public. Health 114, 913–922 (2024).
Joudrey, P. J. et al. A conceptual model for understanding post-release opioid-related overdose risk. Addict. Sci. Clin. Pract. 14, 17 (2019).
Strang, J. Death matters: understanding heroin/opiate overdose risk and testing potential to prevent deaths. Addiction 110, 27–35 (2015).
DuPont, R. L., McLellan, A. T., White, W. L., Merlo, L. J. & Gold, M. S. Setting the standard for recovery: physicians’ health programs. J. Subst. Abus. Treat. 36, 159–171 (2009).
Fuller, R. K. et al. Disulfiram treatment of alcoholism. A Veterans Administration cooperative study. JAMA 256, 1449–1455 (1986).
Chen, B. T. et al. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature 496, 359–362 (2013).
Pascoli, V., Terrier, J., Hiver, A. & Luscher, C. Sufficiency of mesolimbic dopamine neuron stimulation for the progression to addiction. Neuron 88, 1054–1066 (2015).
Durand, A., Girardeau, P., Freese, L. & Ahmed, S. H. Increased responsiveness to punishment of cocaine self-administration after experience with high punishment. Neuropsychopharmacology 47, 444–453 (2022).
Domi, E. et al. Activation of GABA(B) receptors in central amygdala attenuates activity of PKCdelta + neurons and suppresses punishment-resistant alcohol self-administration in rats. Neuropsychopharmacology 48, 1386–1395 (2023).
Giuliano, C., Belin, D. & Everitt, B. J. Compulsive alcohol seeking results from a failure to disengage dorsolateral striatal control over behavior. J. Neurosci. 39, 1744–1754 (2019).
Lee, S. M. et al. Nociceptive stimuli activate the hypothalamus-habenula circuit to inhibit the mesolimbic reward system and cocaine seeking-behaviors. J. Neurosci. 42, 9180–9192 (2022).
McNally, G. P., Jean-Richard-Dit-Bressel, P., Millan, E. Z. & Lawrence, A. J. Pathways to the persistence of drug use despite its adverse consequences. Mol. Psychiatry 28, 2228–2237 (2023).
Pickard, H. & Ahmed, S. H. The Routledge Handbook of Philosophy and Science of Addiction (Routledge, 2018).
Jean-Richard-Dit-Bressel, P., Ma, C., Bradfield, L. A., Killcross, S. & McNally, G. P. Punishment insensitivity emerges from impaired contingency detection, not aversion insensitivity or reward dominance. eLife 8, e52765 (2019).
Marchant, N. J., Khuc, T. N., Pickens, C. L., Bonci, A. & Shaham, Y. Context-induced relapse to alcohol seeking after punishment in a rat model. Biol. Psychiatry 73, 256–262 (2013).
Ghareh, H. et al. Role of anterior insula cortex in context-induced relapse of nicotine-seeking. eLife 11, e75609 (2022).
Sinha, R. Modeling relapse situations in the human laboratory. Curr. Top. Behav. Neurosci. 13, 379–402 (2013).
McDonald, A. J. et al. Alcohol seeking under risk of punishment is associated with activation of cortical and subcortical brain regions. Front. Behav. Neurosci. 15, 739681 (2021).
Choi, E. A. et al. A corticothalamic circuit trades off speed for safety during decision-making under motivational conflict. J. Neurosci. 42, 3473–3483 (2022).
Wang, T. et al. Paraventricular thalamus dynamically modulates aversive memory via tuning prefrontal inhibitory circuitry. J. Neurosci. 43, 3630–3646 (2023).
McNally, G. P. Motivational competition and the paraventricular thalamus. Neurosci. Biobehav. Rev. 125, 193–207 (2021).
Hennigan, K., D’Ardenne, K. & McClure, S. M. Distinct midbrain and habenula pathways are involved in processing aversive events in humans. J. Neurosci. 35, 198–208 (2015).
Hsu, D. T., Kirouac, G. J., Zubieta, J. K. & Bhatnagar, S. Contributions of the paraventricular thalamic nucleus in the regulation of stress, motivation, and mood. Front. Behav. Neurosci. 8, 73 (2014).
Jensen, J. et al. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron 40, 1251–1257 (2003).
Ahmed, S. H. Imbalance between drug and non-drug reward availability: a major risk factor for addiction. Eur. J. Pharmacol. 526, 9–20 (2005).
Venniro, M. et al. The protective effect of social reward on opioid and psychostimulant reward and relapse: behavior, pharmacology, and brain regions. J. Neurosci. 42, 9298–9314 (2022).
Venniro, M., Panlilio, L. V., Epstein, D. H. & Shaham, Y. The protective effect of operant social reward on cocaine self-administration, choice, and relapse is dependent on delay and effort for the social reward. Neuropsychopharmacology 46, 2350–2357 (2021).
Venniro, M. et al. Volitional social interaction prevents drug addiction in rat models. Nat. Neurosci. 21, 1520–1529 (2018).
Grimm, J. W., Hope, B. T., Wise, R. A. & Shaham, Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature 412, 141–142, (2001).
de Boer, S. F., Buwalda, B. & Koolhaas, J. M. Untangling the neurobiology of coping styles in rodents: towards neural mechanisms underlying individual differences in disease susceptibility. Neurosci. Biobehav. Rev. 74, 401–422 (2017).
Krach, S., Paulus, F. M., Bodden, M. & Kircher, T. The rewarding nature of social interactions. Front. Behav. Neurosci. 4, 22 (2010).
Schweinfurth, M. K. The social life of Norway rats (Rattus norvegicus). eLife 9, e54020 (2020).
El Rawas, R. et al. Brain regions associated with the acquisition of conditioned place preference for cocaine vs. social interaction. Front. Behav. Neurosci. 6, 63 (2012).
Leong, K. C., Cox, S., King, C., Becker, H. & Reichel, C. M. Oxytocin and rodent models of addiction. Int. Rev. Neurobiol. 140, 201–247 (2018).
Tomova, L. et al. Acute social isolation evokes midbrain craving responses similar to hunger. Nat. Neurosci. 23, 1597–1605 (2020).
Gunaydin, L. A. et al. Natural neural projection dynamics underlying social behavior. Cell 157, 1535–1551 (2014).
Hung, L. W. et al. Gating of social reward by oxytocin in the ventral tegmental area. Science 357, 1406–1411 (2017).
Dolen, G., Darvishzadeh, A., Huang, K. W. & Malenka, R. C. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501, 179–184 (2013).
Ibragimov, R., Kovacs, G. L., Szabo, G. & Telegdy, G. Microinjection of oxytocin into limbic-mesolimbic brain structures disrupts heroin self-administration behavior: a receptor-mediated event? Life Sci. 41, 1265–1271 (1987).
Weber, R. A. et al. Regionally specific effects of oxytocin on reinstatement of cocaine seeking in male and female rats. Int. J. Neuropsychopharmacol. 21, 677–686 (2018).
Venniro, M. et al. Abstinence-dependent dissociable central amygdala microcircuits control drug craving. Proc. Natl Acad. Sci. USA 117, 8126–8134 (2020).
Domi, E. et al. A neural substrate of compulsive alcohol use. Sci. Adv. 7, eabg9045 (2021).
Marchant, N. J. et al. Rats choose alcohol over social reward in an operant choice procedure. Neuropsychopharmacology 48, 585–593 (2023).
Augier, G. et al. Wistar rats choose alcohol over social interaction in a discrete-choice model. Neuropsychopharmacology 49, 1098–1107 (2022).
Chow, J. J. et al. Characterization of operant social interaction in rats: effects of access duration, effort, peer familiarity, housing conditions, and choice between social interaction vs. food or remifentanil. Psychopharmacology 239, 2093–2108 (2022).
Alexander, B. K., Beyerstein, B. L., Hadaway, P. F. & Coambs, R. B. Effect of early and later colony housing on oral ingestion of morphine in rats. Pharmacol. Biochem. Behav. 15, 571–576 (1981).
Smith, M. A. Peer influences on drug self-administration: social facilitation and social inhibition of cocaine intake in male rats. Psychopharmacology 224, 81–90 (2012).
Papamihali, K. et al. Convenience and comfort: reasons reported for using drugs alone among clients of harm reduction sites in British Columbia, Canada. Harm Reduct. J. 17, 90 (2020).
Roe, L. et al. Isolation, solitude and social distancing for people who use drugs: an ethnographic perspective. Front. Psychiatry 11, 623032 (2020).
Strickland, J. C. & Acuff, S. F. Role of social context in addiction etiology and recovery. Pharmacol. Biochem. Behav. 229, 173603 (2023).
Pelloux, Y., Giorla, E., Montanari, C. & Baunez, C. Social modulation of drug use and drug addiction. Neuropharmacology 159, 107545 (2019).
Mayock, P. & Butler, S. “I’m always hiding and ducking and diving”: the stigma of growing older on methadone. Drugs Educ. Prev. Policy 29, 139–149 (2022).
Engeln, M., Fox, M. E. & Lobo, M. K. Housing conditions during self-administration determine motivation for cocaine in mice following chronic social defeat stress. Psychopharmacology 238, 41–54 (2021).
Heilig, M., Epstein, D. H., Nader, M. A. & Shaham, Y. Time to connect: bringing social context into addiction neuroscience. Nat. Rev. Neurosci. 17, 592–599 (2016).
Scheggia, D. et al. Reciprocal cortico-amygdala connections regulate prosocial and selfish choices in mice. Nat. Neurosci. 25, 1505–1518 (2022).
Rabin, R. A., Parvaz, M. A., Alia-Klein, N. & Goldstein, R. Z. Emotion recognition in individuals with cocaine use disorder: the role of abstinence length and the social brain network. Psychopharmacology 239, 1019–1033 (2022).
Chang, V. N. & Peters, J. Neural circuits controlling choice behavior in opioid addiction. Neuropharmacology 226, 109407 (2023).
Lenoir, M. et al. Large-scale brain correlates of sweet versus cocaine reward in rats. Eur. J. Neurosci. 57, 423–439 (2023).
Haubrich, J. & Nader, K. in Behavioral Neuroscience of Learning and Memory (eds Clark, R. E. & Martin, S. J.) 151–176 (Springer International Publishing, 2018).
Bernabo, M., Haubrich, J., Gamache, K. & Nader, K. Memory destabilization and reconsolidation dynamically regulate the PKMζ maintenance mechanism. J. Neurosci. 41, 4880–4888 (2021).
Huang, Y. H. et al. In vivo cocaine experience generates silent synapses. Neuron 63, 40–47 (2009).
Wright, W. J. & Dong, Y. Silent synapses in cocaine-associated memory and beyond. J. Neurosci. 41, 9275–9285 (2021).
Engeln, M. et al. Transcriptome profiling of the ventral pallidum reveals a role for pallido-thalamic neurons in cocaine reward. Mol. Psychiatry 27, 3980–3991 (2022).
Finkelstein, A. B. et al. Social reactivation of fear engrams enhances memory recall. Proc. Natl Acad. Sci. USA 119, e2114230119 (2022).
Smith, K. E. Disease and decision. J. Subst. Abus. Treat. 142, 108874 (2022).
Lim, D. H., Yoon, Y. J., Her, E., Huh, S. & Jung, M. W. Active maintenance of eligibility trace in rodent prefrontal cortex. Sci. Rep. 10, 18860 (2020).
Liu, X. et al. Memory consolidation drives the enhancement of remote cocaine memory via prefrontal circuit. Mol. Psychiatry 29, 730–741 (2024).
Kober, H. et al. Prefrontal–striatal pathway underlies cognitive regulation of craving. Proc. Natl Acad. Sci. USA 107, 14811–14816 (2010).
Rescorla, R. A. & Wagner, A. R. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and non-reinforcement. In Classical Conditioning: Current Research and Theory Vol. 2 (eds Black, A. H. & Prokasy, W. F.) 64–69 (Appleton-Century-Crofts, 1972).
Pedreira, M. E., Perez-Cuesta, L. M. & Maldonado, H. Mismatch between what is expected and what actually occurs triggers memory reconsolidation or extinction. Learn. Mem. 11, 579–585, (2004).
Kendler, K. S. & Woodward, J. Top-down causation in psychiatric disorders: a clinical-philosophical inquiry. Psychol. Med. 51, 1783–1788 (2021).
Milton, A. L. & Everitt, B. J. The psychological and neurochemical mechanisms of drug memory reconsolidation: implications for the treatment of addiction. Eur. J. Neurosci. 31, 2308–2319, (2010).
Pachas, G. N. et al. Single dose propranolol does not affect physiologic or emotional reactivity to smoking cues. Psychopharmacology 232, 1619–1628 (2015).
Xue, Y. X. et al. Effect of selective inhibition of reactivated nicotine-associated memories with propranolol on nicotine craving. JAMA Psychiatry 74, 224–232, (2017).
Lonergan, M. et al. Reactivating addiction-related memories under propranolol to reduce craving: a pilot randomized controlled trial. J. Behav. Ther. Exp. Psychiatry 50, 245–249 (2016).
Jobes, M. L. et al. Effects of prereactivation propranolol on cocaine craving elicited by imagery script/cue sets in opioid-dependent polydrug users: a randomized study. J. Addict. Med. 9, 491–498 (2015).
Bornstein, A. M. & Pickard, H. “Chasing the first high”: memory sampling in drug choice. Neuropsychopharmacology 45, 907–915 (2020).
Davis, R. L. & Zhong, Y. The biology of forgetting—a perspective. Neuron 95, 490–503 (2017).
Gallo, F. T. et al. Dopamine modulates adaptive forgetting in medial prefrontal cortex. J. Neurosci. 42, 6620–6636 (2022).
Kerchner, G. A. & Nicoll, R. A. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat. Rev. Neurosci. 9, 813–825 (2008).
Rogerson, T. et al. Synaptic tagging during memory allocation. Nat. Rev. Neurosci. 15, 157–169 (2014).
Cai, D. J. et al. A shared neural ensemble links distinct contextual memories encoded close in time. Nature 534, 115–118 (2016).
Yetton, B. D., Cai, D. J., Spoormaker, V. I., Silva, A. J. & Mednick, S. C. Human memories can be linked by temporal proximity. Front. Hum. Neurosci. 13, 315 (2019).
Koob, G. F., Sanna, P. P. & Bloom, F. E. Neuroscience of addiction. Neuron 21, 467–476 (1998).
Koob, G. F. & Volkow, N. D. Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238 (2010).
Schlag, A. K. Percentages of problem drug use and their implications for policy making: a review of the literature. Drug. Sci. Policy Law 6, 2050324520904540 (2020).
Schultz, W. Updating dopamine reward signals. Curr. Opin. Neurobiol. 23, 229–238 (2013).
Piantadosi, P. T., Yeates, D. C. M., Wilkins, M. & Floresco, S. B. Contributions of basolateral amygdala and nucleus accumbens subregions to mediating motivational conflict during punished reward-seeking. Neurobiol. Learn. Mem. 140, 92–105 (2017).
Leong, K. C. et al. Oxytocin reduces cocaine cued Fos activation in a regionally specific manner. Int. J. Neuropsychopharmacol. 20, 844–854 (2017).
Lenoir, M., Engeln, M., Navailles, S., Girardeau, P. & Ahmed, S. H. A large-scale c-Fos brain mapping study on extinction of cocaine-primed reinstatement. Neuropsychopharmacology 49, 1459–1467 (2024).
Pelloux, Y., Minier-Toribio, A., Hoots, J. K., Bossert, J. M. & Shaham, Y. Opposite effects of basolateral amygdala inactivation on context-induced relapse to cocaine seeking after extinction versus punishment. J. Neurosci. 38, 51–59 (2018).
Diniz, C. & Crestani, A. P. The times they are a-changin’: a proposal on how brain flexibility goes beyond the obvious to include the concepts of “upward” and “downward” to neuroplasticity. Mol. Psychiatry 28, 977–992 (2023).
Bozon, B., Davis, S. & Laroche, S. A requirement for the immediate early gene zif268 in reconsolidation of recognition memory after retrieval. Neuron 40, 695–701 (2003).
Chandra, R. et al. Optogenetic inhibition of D1R containing nucleus accumbens neurons alters cocaine-mediated regulation of Tiam1. Front. Mol. Neurosci. 6, 13 (2013).
Tu, G. et al. Dopamine D1 and D2 receptors differentially regulate Rac1 and Cdc42 signaling in the nucleus accumbens to modulate behavioral and structural plasticity after repeated methamphetamine treatment. Biol. Psychiatry 86, 820–835 (2019).
Roesler, R. Molecular mechanisms controlling protein synthesis in memory reconsolidation. Neurobiol. Learn. Mem. 142, 30–40 (2017).
Barak, S. & Goltseker, K. Targeting the reconsolidation of licit drug memories to prevent relapse: focus on alcohol and nicotine. Int. J. Mol. Sci. 22, 4090 (2021).
Chamberlain, S. R., Muller, U., Blackwell, A. D., Robbins, T. W. & Sahakian, B. J. Noradrenergic modulation of working memory and emotional memory in humans. Psychopharmacology 188, 397–407 (2006).
Otis, J. M. & Mueller, D. Reversal of cocaine-associated synaptic plasticity in medial prefrontal cortex parallels elimination of memory retrieval. Neuropsychopharmacology 42, 2000–2010 (2017).
Akirav, I. & Maroun, M. Stress modulation of reconsolidation. Psychopharmacology 226, 747–761, (2013).
Haubrich, J., Bernabo, M. & Nader, K. Noradrenergic projections from the locus coeruleus to the amygdala constrain fear memory reconsolidation. eLife 9, e57010 (2020).
Sanchez Beisel, J. M., Maza, F. J., Justel, N., Fernandez Larrosa, P. N. & Delorenzi, A. Embodiment of an emotional state concurs with a stress-induced reconsolidation impairment effect on an auditory verbal word-list memory. Neuroscience 497, 239–256 (2022).
Goltseker, K., Handrus, H. & Barak, S. Disruption of relapse to alcohol seeking by aversive counterconditioning following memory retrieval. Addict. Biol. 26, e12935 (2021).
Goltseker, K., Bolotin, L. & Barak, S. Counterconditioning during reconsolidation prevents relapse of cocaine memories. Neuropsychopharmacology 42, 716–726 (2017).
Acknowledgements
This work was supported by the French Research Council (CNRS), the Université de Bordeaux, the French National Agency (ANR-22-CE37-0004 to M.E. and ANR-19-CE37-0013 to S.H.A.) and IReSP/INCa (SPAV1-22-003 to S.H.A.).
Author information
Authors and Affiliations
Contributions
M.E. researched data for the article. Both authors contributed substantially to discussion of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Peer review
Peer review information
Nature Reviews Neuroscience thanks Yan Dong, Yavin Shaham, Yingjie Zhu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Engeln, M., Ahmed, S.H. Remission from addiction: erasing the wrong circuits or making new ones?. Nat. Rev. Neurosci. 26, 115–130 (2025). https://doi.org/10.1038/s41583-024-00886-y
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41583-024-00886-y