Abstract
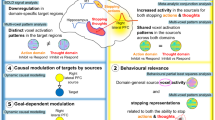
Controlling action and thought requires the capacity to stop mental processes. Over the past two decades, evidence has grown that a domain-general inhibitory control mechanism supported by the right lateral prefrontal cortex achieves these functions. However, current views of the neural mechanisms of inhibitory control derive largely from research into the stopping of action. Whereas action stopping is a convenient empirical model, it does not invoke thought inhibition and cannot be used to identify the unique features of this process. Here, we review research that addresses how organisms stop a key process that drives thoughts: memory retrieval. This work has shown that retrieval stopping shares right dorsolateral and ventrolateral prefrontal mechanisms with action stopping, consistent with a domain-general inhibitory control mechanism, but also recruits a distinct fronto-temporal pathway that determines the success of mental control. As part of this pathway, GABAergic inhibition within the hippocampus influences the efficacy of prefrontal control over thought. These unique elements of mental control suggest that hippocampal disinhibition is a transdiagnostic factor underlying intrusive thinking, linking the fronto-temporal control pathway to preclinical models of psychiatric disorders and fear extinction. We suggest that retrieval-stopping deficits may underlie the intrusive thinking that is common across many psychiatric disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Falconer, E. et al. The neural networks of inhibitory control in posttraumatic stress disorder. J. Psychiatry Neurosci. 33, 413–422 (2008).
Visser, R. et al. in Intrusive Thinking: From Molecules to Free Will (eds Kalivas, P. W. & Paulus, M. P.) 124–184 (MIT Press, 2020). Results of a conference on intrusive thinking attended by 40 scientists in multiple areas of psychology and neuroscience, capturing multiple perspectives on the topic.
Kalivas, P. W., Gourley, S. L. & Paulus, M. P. Intrusive thinking: circuit and synaptic mechanisms of a transdiagnostic psychiatric symptom. Neurosci. Biobehav. Rev. 150, 105196 (2023).
Watkins, E. R. Constructive and unconstructive repetitive thought. Psychol. Bull. 134, 163–206 (2008).
Payne, A., Kralj, A., Young, J. & Meiser-Stedman, R. The prevalence of intrusive memories in adult depression: a meta-analysis. J. Affect. Disord. 253, 193–202 (2019).
Badcock, J. C. & Hugdahl, K. Cognitive mechanisms of auditory verbal hallucinations in psychotic and non-psychotic groups. Neurosci. Biobehav. Rev. 36, 431–438 (2012).
Alderson-Day, B. et al. Intentional inhibition but not source memory is related to hallucination-proneness and intrusive thoughts in a university sample. Cortex 113, 267–278 (2019).
Clark, D. A. Intrusive Thoughts in Clinical Disorders: Theory, Research, and Treatment (Guilford, 2005).
Aron, A. R. The neural basis of inhibition in cognitive control. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 13, 214–228 (2007).
Jahanshahi, M., Obeso, I., Rothwell, J. C. & Obeso, J. A. A fronto–striato–subthalamic–pallidal network for goal-directed and habitual inhibition. Nat. Rev. Neurosci. 16, 719–732 (2015).
Lipszyc, J. & Schachar, R. Inhibitory control and psychopathology: a meta-analysis of studies using the stop signal task. J. Int. Neuropsychol. Soc. 16, 1064–1076 (2010).
Logan, G. D. & Cowan, W. B. On the ability to inhibit thought and action: a theory of an act of control. Psychol. Rev. 91, 295–327 (1984).
Logan, G. D., Van Zandt, T., Verbruggen, F. & Wagenmakers, E.-J. On the ability to inhibit thought and action: general and special theories of an act of control. Psychol. Rev. 121, 66–95 (2014).
Matzke, D., Verbruggen, F. & Logan, G. D. in Stevens’ Handbook of Experimental Psychology and Cognitive Neuroscience (ed. Wixted, J. T.) 1–45 (Wiley, 2018).
Schall, J. D., Palmeri, T. J. & Logan, G. D. Models of inhibitory control. Philos. Trans. R. Soc. B Biol. Sci. 372, 20160193 (2017).
Aron, A. R., Robbins, T. W. & Poldrack, R. A. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn. Sci. 18, 177–185 (2014).
Bari, A. & Robbins, T. W. Inhibition and impulsivity: behavioral and neural basis of response control. Prog. Neurobiol. 108, 44–79 (2013).
Wessel, J. R. & Anderson, M. C. Neural mechanisms of domain-general inhibitory control. Trends Cogn. Sci. 28, 124–143 (2024). A review of evidence for a domain-general inhibitory control mechanism with a novel proposal about the role of thalamic drive reduction.
Apšvalka, D., Ferreira, C. S., Schmitz, T. W., Rowe, J. B. & Anderson, M. C. Dynamic targeting enables domain-general inhibitory control over action and thought by the prefrontal cortex. Nat. Commun. 13, 274 (2022). Important evidence for a domain-general inhibitory control process in right dorsolateral and ventrolateral prefrontal cortices that dynamically shifts connectivity between motor cortex and hippocampus, depending on whether actions or thoughts need to be stopped.
Wimber, M., Alink, A., Charest, I., Kriegeskorte, N. & Anderson, M. C. Retrieval induces adaptive forgetting of competing memories via cortical pattern suppression. Nat. Neurosci. 18, 582–589 (2015).
Kim, H., Smolker, H. R., Smith, L. L., Banich, M. T. & Lewis-Peacock, J. A. Changes to information in working memory depend on distinct removal operations. Nat. Commun. 11, 6239 (2020).
Brewin, C. R., Gregory, J. D., Lipton, M. & Burgess, N. Intrusive images in psychological disorders: characteristics, neural mechanisms, and treatment implications. Psychol. Rev. 117, 210–232 (2010).
Levy, B. J. & Anderson, M. C. Purging of memories from conscious awareness tracked in the human brain. J. Neurosci. 32, 16785–16794 (2012). An early demonstration that intrusions of unwanted thoughts trigger increased suppression of hippocampal activity, indicating that hippocampal downregulation reflects reactive control.
Mace, J. H. in Involuntary Memory (ed. Mace, J. H.) 1–19 (Blackwell Publishing, 2007).
Berntsen, D. Involuntary autobiographical memories and their relation to other forms of spontaneous thoughts. Philos. Trans. R. Soc. B Biol. Sci. 376, 20190693 (2020).
Aron, A. R., Robbins, T. W. & Poldrack, R. A. Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 8, 170–177 (2004).
Anderson, M. C. & Weaver, C. in Encyclopedia of Neuroscience (ed. Squire, L. R.) 153–163 (Academic, 2009).
Renoult, L., Irish, M., Moscovitch, M. & Rugg, M. D. From knowing to remembering: the semantic–episodic distinction. Trends Cogn. Sci. 23, 1041–1057 (2019).
Tanguay, A. F. et al. The shared and unique neural correlates of personal semantic, general semantic, and episodic memory. eLife 12, e83645 (2023).
Anderson, M. C. & Green, C. Suppressing unwanted memories by executive control. Nature 410, 366–369 (2001). An early demonstration that intentionally stopping the retrieval process induces forgetting of suppressed thoughts, consistent with inhibitory control.
Nardo, D. & Anderson, M. C. Everything you ever wanted to know about the think/no-think task, but forgot to ask. Behav. Res. Methods 56, 3831–3860 (2024).
Benoit, R. G. & Anderson, M. C. Opposing mechanisms support the voluntary forgetting of unwanted memories. Neuron 76, 450–460 (2012). Demonstrates two distinct mechanisms for controlling unwanted memories, thought substitution and direct suppression, with opposite effects on hippocampal activity and relying on distinct prefrontal mechanisms.
Hertel, P. T. & Calcaterra, G. Intentional forgetting benefits from thought substitution. Psychon. Bull. Rev. 12, 484–489 (2005).
Bergström, Z. M., De Fockert, J. W. & Richardson-Klavehn, A. ERP and behavioural evidence for direct suppression of unwanted memories. NeuroImage 48, 726–737 (2009). A pioneering article that dissociates direct suppression and thought substitution as distinct mechanisms of memory control.
Aron, A. R. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol. Psychiatry 69, e55–e68 (2011).
Munakata, Y. et al. A unified framework for inhibitory control. Trends Cogn. Sci. 15, 453–459 (2011).
Bissett, P. G. & Logan, G. D. Selective stopping? Maybe not. J. Exp. Psychol. Gen. 143, 455–472 (2014).
Duque, J., Greenhouse, I., Labruna, L. & Ivry, R. B. Physiological markers of motor inhibition during human behavior. Trends Neurosci. 40, 219–236 (2017).
Tarder-Stoll, H., Jayakumar, M., Dimsdale-Zucker, H. R., Günseli, E. & Aly, M. Dynamic internal states shape memory retrieval. Neuropsychologia 138, 107328 (2020).
Madore, K. P. & Wagner, A. D. Readiness to remember: predicting variability in episodic memory. Trends Cogn. Sci. 26, 707–723 (2022).
Anderson, M. C. & Hulbert, J. C. Active forgetting: adaptation of memory by prefrontal control. Annu. Rev. Psychol. 72, 1–36 (2021). An integrated review of research demonstrating that humans engage prefrontal control processes to adapt the state of memory to cognitive and emotional goals, inducing active forgetting.
Levy, B. J. & Anderson, M. C. Individual differences in the suppression of unwanted memories: the executive deficit hypothesis. Acta Psychol. 127, 623–635 (2008).
Stramaccia, D. F., Meyer, A.-K., Rischer, K. M., Fawcett, J. M. & Benoit, R. G. Memory suppression and its deficiency in psychological disorders: a focused meta-analysis. J. Exp. Psychol. Gen. 150, 828–850 (2021). Meta-analytic evidence that a range of psychological disorders are associated with impaired suppression-induced forgetting, relative to control samples.
Wang, Y., Cao, Z., Zhu, Z., Cai, H. & Wu, Y. Cue-independent forgetting by intentional suppression — evidence for inhibition as the mechanism of intentional forgetting. Cognition 143, 31–35 (2015).
Liu, P. et al. Task compliance predicts suppression-induced forgetting in a large sample. Sci. Rep. 11, 20166 (2021).
Wang, Y. & Zhu, Z. Retrieval suppression induced forgetting on 1-week-old consolidated episodic memories. Psychon. Bull. Rev. 29, 1377–1386 (2022).
Schmidt, M., Anderson, M. C. & Tempel, T. Suppression-induced forgetting of motor sequences. Cognition 230, 105292 (2023).
Kim, D.-Y., Oh, D. H., Kim, S. H., Sim, K.-B. & Lee, J.-H. Effects of intentional suppression of recall of unwanted images in repressors and nonrepressors. Soc. Behav. Pers. Int. J. 41, 319–326 (2013).
Anderson, M. C. & Hanslmayr, S. Neural mechanisms of motivated forgetting. Trends Cogn. Sci. 18, 279–292 (2014).
Engen, H. G. & Anderson, M. C. Memory control: a fundamental mechanism of emotion regulation. Trends Cogn. Sci. 22, 982–995 (2018). A synthesis of work arguing that stopping upsetting thoughts provides a vital mechanism for regulating emotion, with a consideration of how conventional emotion regulation strategies may recruit suppression.
Küpper, C. S., Benoit, R. G., Dalgleish, T. & Anderson, M. C. Direct suppression as a mechanism for controlling unpleasant memories in daily life. J. Exp. Psychol. Gen. 143, 1443–1449 (2014).
Marsh, L. C. & Anderson, M. C. in The Oxford Handbook of Human Memory, Two Volume Pack (eds Kahana, M. J. & Wagner, A. D.) 1209–1256 (Oxford Univ. Press, 2024).
López-Caneda, E., Crego, A., Campos, A. D., González-Villar, A. & Sampaio, A. The think/no-think alcohol task: a new paradigm for assessing memory suppression in alcohol-related contexts. Alcohol Clin. Exp. Res. 43, 36–47 (2019).
Hu, X., Bergström, Z. M., Bodenhausen, G. V. & Rosenfeld, J. P. Suppressing unwanted autobiographical memories reduces their automatic influences: evidence from electrophysiology and an implicit autobiographical memory test. Psychol. Sci. 26, 1098–1106 (2015).
Noreen, S. & Macleod, M. D. It’s all in the detail: intentional forgetting of autobiographical memories using the autobiographical think/no-think task. J. Exp. Psychol. Learn. Mem. Cogn. 39, 375–393 (2013).
Lu, F., Yang, W. & Qiu, J. Neural bases of motivated forgetting of autobiographical memories. Cogn. Neurosci. 14, 15–24 (2023).
Waldhauser, G. T., Lindgren, M. & Johansson, M. Intentional suppression can lead to a reduction of memory strength: behavioral and electrophysiological findings. Front. Psychol. 3, 401 (2012).
Benoit, R. G., Hulbert, J. C., Huddleston, E. & Anderson, M. C. Adaptive top-down suppression of hippocampal activity and the purging of intrusive memories from consciousness. J. Cogn. Neurosci. 27, 96–111 (2015).
Gagnepain, P., Hulbert, J. & Anderson, M. C. Parallel regulation of memory and emotion supports the suppression of intrusive memories. J. Neurosci. 37, 6423–6441 (2017). Evidence showing that stopping retrieval of unpleasant scenes regulates both memory and affect by parallel modulation of the hippocampus and amygdala, driven by intrusions.
Harrington, M. O., Ashton, J. E., Sankarasubramanian, S., Anderson, M. C. & Cairney, S. A. Losing control: sleep deprivation impairs the suppression of unwanted thoughts. Clin. Psychol. Sci. 9, 97–113 (2021).
Mary, A. et al. Resilience after trauma: the role of memory suppression. Science 367, eaay8477 (2020). Compelling demonstration of how fronto-hippocampal modulation during thought suppression, particularly in response to intrusions, plays a critical role in resilience, in the context of the November 15th Paris terrorist attacks.
van Schie, K. & Anderson, M. C. Successfully controlling intrusive memories is harder when control must be sustained. Memory 25, 1201–1216 (2017).
Legrand, N. et al. Attentional capture mediates the emergence and suppression of intrusive memories. iScience 25, 105516 (2022). Innovative use of pattern classification on electroencephalography time–frequency data to identify the occurrence and timing of intrusions during retrieval suppression and their engagement of attentional capture mechanisms.
Legrand, N. et al. Long-term modulation of cardiac activity induced by inhibitory control over emotional memories. Sci. Rep. 10, 1–19 (2020).
Hellerstedt, R., Johansson, M. & Anderson, M. C. Tracking the intrusion of unwanted memories into awareness with event-related potentials. Neuropsychologia 89, 510–523 (2016).
Gagnepain, P., Henson, R. N. & Anderson, M. C. Suppressing unwanted memories reduces their unconscious influence via targeted cortical inhibition. Proc. Natl Acad. Sci. USA 111, E1310–E1319 (2014). An early demonstration that stopping retrieval suppresses both hippocampal and neocortical regions in parallel, inducing a persisting cortical aftereffect that disrupts unconscious memory.
Kim, K. & Yi, D.-J. Out of mind, out of sight: perceptual consequences of memory suppression. Psychol. Sci. 24, 569–574 (2013). A pioneering study that demonstrated how suppressing visual memories impaired later perception of the suppressed content.
Hertel, P. T., Large, D., Stück, E. D. & Levy, A. Suppression-induced forgetting on a free-association test. Memory 20, 100–109 (2012).
Taubenfeld, A., Anderson, M. C. & Levy, D. A. The impact of retrieval suppression on conceptual implicit memory. Memory 27, 686–697 (2018).
Wang, Y., Luppi, A., Fawcett, J. & Anderson, M. C. Reconsidering unconscious persistence: suppressing unwanted memories reduces their indirect expression in later thoughts. Cognition 187, 78–94 (2019).
Hertel, P. T., Maydon, A., Ogilvie, A. & Mor, N. Ruminators (unlike others) fail to show suppression-induced forgetting on indirect measures of memory. Clin. Psychol. Sci. 6, 872–881 (2018).
Hu, X., Bergström, Z. M., Gagnepain, P. & Anderson, M. C. Suppressing unwanted memories reduces their unintended influences. Curr. Dir. Psychol. Sci. 26, 197–206 (2017).
Whitlock, J., Lo, Y.-P., Chiu, Y.-C. & Sahakyan, L. Eye movement analyses of strong and weak memories and goal-driven forgetting. Cognition 204, 104391 (2020).
Kulkarni, M., Nickel, A. E., Minor, G. N. & Hannula, D. E. Control of memory retrieval alters memory-based eye movements. J. Exp. Psychol. Learn. Mem. Cogn. 50, 1199–1219 (2023).
Anderson, M. C., Bunce, J. G. & Barbas, H. Prefrontal–hippocampal pathways underlying inhibitory control over memory. Neurobiol. Learn. Mem. 134, 145–161 (2016). Discusses pathways by which the prefrontal cortex could modulate hippocampal activity during retrieval stopping, based on detailed review of primate and rodent anatomical studies, and introduces the entorhinal gating and thalamo-hippocampal modulation hypotheses.
Anderson, M. C. et al. Neural systems underlying the suppression of unwanted memories. Science 303, 232–235 (2004). Introduces our fronto-hippocampal model of motivated forgetting, demonstrating the engagement of right dorsolateral prefrontal cortex and the suppression of hippocampal activity in helping us to forget unwanted thoughts.
Crespo-García, M., Wang, Y., Jiang, M., Anderson, M. C. & Lei, X. Anterior cingulate cortex signals the need to control intrusive thoughts during motivated forgetting. J. Neurosci. 42, 4342–4359 (2022). A rare simultaneous functional MRI/electroencephalography study revealing the dynamic interaction between the anterior cingulate cortex, which detects intrusive thoughts, and the right dorsolateral prefrontal cortex, to upregulate control by increasing hippocampal suppression via beta-oscillatory signals.
Schmitz, T. W., Correia, M. M., Ferreira, C. S., Prescot, A. P. & Anderson, M. C. Hippocampal GABA enables inhibitory control over unwanted thoughts. Nat. Commun. 8, 1311 (2017). Evidence that retrieval stopping engages GABAergic interneurons in the hippocampus to downregulate its activity and disrupt retention, implementing key aspects of inhibitory control.
Sacchet, M. D. et al. Cognitive and neural consequences of memory suppression in major depressive disorder. Cogn. Affect. Behav. Neurosci. 17, 77–93 (2017).
Paz-Alonso, P. M., Bunge, S. A., Anderson, M. C. & Ghetti, S. Strength of coupling within a mnemonic control network differentiates those who can and cannot suppress memory retrieval. J. Neurosci. 33, 5017–5026 (2013).
Depue, B. E., Curran, T. & Banich, M. T. Prefrontal regions orchestrate suppression of emotional memories via a two-phase process. Science 317, 215–219 (2007). An early demonstration that retrieval stopping mechanisms could suppress the retrieval of aversive memories by modulation of the hippocampus and amygdala.
Depue, B. E., Orr, J. M., Smolker, H. R., Naaz, F. & Banich, M. T. The organization of right prefrontal networks reveals common mechanisms of inhibitory regulation across cognitive, emotional, and motor processes. Cereb. Cortex 26, 1634 (2016). Evidence for a region in the right dorsolateral prefrontal cortex mediating the stopping of actions, thoughts and affective responses.
Liu, Y. et al. Memory consolidation reconfigures neural pathways involved in the suppression of emotional memories. Nat. Commun. 7, 13375 (2016).
Meyer, A.-K. & Benoit, R. G. Suppression weakens unwanted memories via a sustained reduction of neural reactivation. eLife 11, e71309 (2022). Evidence that retrieval stopping weakens representations of suppressed scenes, as reflected by reduced scene information in the parahippocampal place area both during suppression and on later retrieval tests for suppressed content.
Sullivan, D. R. et al. Behavioral and neural correlates of memory suppression in PTSD. J. Psychiatr. Res. 112, 30–37 (2019).
Banich, M. T., Mackiewicz Seghete, K. L., Depue, B. E. & Burgess, G. C. Multiple modes of clearing one’s mind of current thoughts: overlapping and distinct neural systems. Neuropsychologia 69, 105–117 (2015).
DeRosa, J., Kim, H., Lewis-Peacock, J. & Banich, M. T. Neural systems underlying the implementation of working memory removal operations. J. Neurosci. 44, e0283232023 (2024).
Nee, D. E. et al. A meta-analysis of executive components of working memory. Cereb. Cortex 23, 264–282 (2013).
Lewis-Peacock, J. A., Kessler, Y. & Oberauer, K. The removal of information from working memory. Ann. N. Y. Acad. Sci. 1424, 33–44 (2018).
Diamond, A. Executive functions. Annu. Rev. Psychol. 64, 135–168 (2013).
Kane, M. J. & Engle, R. W. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychon. Bull. Rev. 9, 637–671 (2002).
Kane, M. J., Bleckley, M. K., Conway, A. R. & Engle, R. W. A controlled-attention view of working-memory capacity. J. Exp. Psychol. Gen. 130, 169–183 (2001).
Wierzba, M. et al. Cognitive control over memory — individual differences in memory performance for emotional and neutral material. Sci. Rep. 8, 3808 (2018).
Eich, T. S., Lao, P. & Anderson, M. C. Cortical thickness in the right inferior frontal gyrus mediates age-related performance differences on an item-method directed forgetting task. Neurobiol. Aging 106, 95–102 (2021).
Mitchell, J. P. et al. Separating sustained from transient aspects of cognitive control during thought suppression. Psychol. Sci. 18, 292–297 (2007).
Koenig-Robert, R. & Pearson, J. Decoding nonconscious thought representations during successful thought suppression. J. Cogn. Neurosci. 32, 2272–2284 (2020).
Conway, M. & Fthenaki, A. Disruption of inhibitory control of memory following lesions to the frontal and temporal lobes. Cortex 39, 667–686 (2003).
Jamadar, S. D., Fielding, J. & Egan, G. F. Quantitative meta-analysis of fMRI and PET studies reveals consistent activation in fronto-striatal-parietal regions and cerebellum during antisaccades and prosaccades. Front. Psychol. 4, 749 (2013).
Coe, B. C. & Munoz, D. P. Mechanisms of saccade suppression revealed in the anti-saccade task. Philos. Trans. R. Soc. B Biol. Sci. 372, 20160192 (2017).
Coiner, B. et al. Functional neuroanatomy of the human eye movement network: a review and atlas. Brain Struct. Funct. 224, 2603–2617 (2019).
Gavazzi, G. et al. Subregional prefrontal cortex recruitment as a function of inhibitory demand: an fMRI metanalysis. Neurosci. Biobehav. Rev. 152, 105285 (2023).
Fedorenko, E., Duncan, J. & Kanwisher, N. Broad domain generality in focal regions of frontal and parietal cortex. Proc. Natl Acad. Sci. USA 110, 16616–16621 (2013).
Assem, M., Shashidhara, S., Glasser, M. F. & Duncan, J. Basis of executive functions in fine-grained architecture of cortical and subcortical human brain networks. Cereb. Cortex 34, bhad537 (2024).
Guo, Y., Schmitz, T. W., Mur, M., Ferreira, C. S. & Anderson, M. C. A supramodal role of the basal ganglia in memory and motor inhibition: meta-analytic evidence. Neuropsychologia 108, 117–134 (2018). A meta-analysis with convincing evidence for a role of the basal ganglia in retrieval stopping, and the colocalization of retrieval and action stopping activations.
Hannah, R. & Aron, A. R. Towards real-world generalizability of a circuit for action-stopping. Nat. Rev. Neurosci. 22, 538–552 (2021).
Depue, B. E., Burgess, G. C., Willcutt, E. G., Ruzic, L. & Banich, M. T. Inhibitory control of memory retrieval and motor processing associated with the right lateral prefrontal cortex: evidence from deficits in individuals with ADHD. Neuropsychologia 48, 3909–3917 (2010).
Castiglione, A., Wagner, J., Anderson, M. & Aron, A. R. Preventing a thought from coming to mind elicits increased right frontal beta just as stopping action does. Cereb. Cortex 29, 2160–2172 (2019).
Mecklinger, A., Parra, M. & Waldhauser, G. T. ERP correlates of intentional forgetting. Brain Res. 1255, 132–147 (2009).
Streb, M., Mecklinger, A., Anderson, M. C., Johanna, L. H. & Michael, T. Memory control ability modulates intrusive memories after analogue trauma. J. Affect. Disord. 192, 134–142 (2016).
Chen, S., Mao, X. & Wu, Y. Can’t stop thinking: the role of cognitive control in suppression-induced forgetting. Neuropsychologia 172, 108274 (2022).
Subbulakshmi, S. Domain-General Control Mechanisms Underlying Stopping of Thought and Action. Doctoral thesis, University of Cambridge (2022).
Gillie, B. L., Vasey, M. W. & Thayer, J. F. Heart rate variability predicts control over memory retrieval. Psychol. Sci. 25, 458–465 (2014).
Gunduz, T., Gunduz, H. & Cetinkaya, H. Increase in physiological inhibitory control results in better suppression of unwanted memories. Br. J. Psychol. 114, 908–927 (2023).
Hubbard, R. J. & Sahakyan, L. Differential recruitment of inhibitory control processes by directed forgetting and thought substitution. J. Neurosci. 43, 1963–1975 (2023).
Oehrn, C. R. et al. Direct electrophysiological evidence for prefrontal control of hippocampal processing during voluntary forgetting. Curr. Biol. 28, 3016–3022.e4 (2018). A rare glimpse of the electrophysiological mechanisms underlying intentional forgetting using intracranial recording in the human hippocampus and prefrontal cortex, providing temporally, spatially and mechanistically specific evidence for top-down control of memory.
Smolker, H. R., Friedman, N. P., Hewitt, J. K. & Banich, M. T. Neuroanatomical correlates of the unity and diversity model of executive function in young adults. Front. Hum. Neurosci. 12, 283 (2018).
Friedman, N. P. & Robbins, T. W. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology 47, 72–89 (2022).
Badre, D. in The Frontal Cortex (eds Banich, M. T., Haber, S. N. & Robbins, T. W.) 109–130 (The MIT Press, 2024).
Schachar, R. et al. Restraint and cancellation: multiple inhibition deficits in attention deficit hyperactivity disorder. J. Abnorm. Child Psychol. 35, 229–238 (2007).
Verbruggen, F. & Logan, G. D. Response inhibition in the stop-signal paradigm. Trends Cogn. Sci. 12, 418–424 (2008).
Dalley, J. W., Everitt, B. J. & Robbins, T. W. Impulsivity, compulsivity, and top-down cognitive control. Neuron 69, 680–694 (2011).
Raud, L., Westerhausen, R., Dooley, N. & Huster, R. J. Differences in unity: the go/no-go and stop signal tasks rely on different mechanisms. NeuroImage 210, 116582 (2020).
Swick, D., Ashley, V. & Turken, U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. NeuroImage 56, 1655–1665 (2011).
Zhang, R., Geng, X. & Lee, T. M. C. Large-scale functional neural network correlates of response inhibition: an fMRI meta-analysis. Brain Struct. Funct. 222, 3973–3990 (2017).
Cipolotti, L. et al. Inhibition processes are dissociable and lateralized in human prefrontal cortex. Neuropsychologia 93, 1–12 (2016).
Okayasu, M. et al. The Stroop effect involves an excitatory–inhibitory fronto-cerebellar loop. Nat. Commun. 14, 27 (2023).
Bartolomeo, P. & Seidel Malkinson, T. Hemispheric lateralization of attention processes in the human brain. Curr. Opin. Psychol. 29, 90–96 (2019).
Parris, B. A. et al. An fMRI study of response and semantic conflict in the Stroop task. Front. Psychol. 10, 2426 (2019).
Huang, Y., Su, L. & Ma, Q. The Stroop effect: an activation likelihood estimation meta-analysis in healthy young adults. Neurosci. Lett. 716, 134683 (2020).
Noreen, S. & MacLeod, M. D. What do we really know about cognitive inhibition? Task demands and inhibitory effects across a range of memory and behavioural tasks. PLoS ONE 10, e0134951 (2015).
Anderson, M. C. & Levy, B. J. in Inhibition in Cognition (eds Gorfein, D. S. & MacLeod, C. M.) 81–102 (American Psychological Association, 2007).
Schilling, C. J., Storm, B. C. & Anderson, M. C. Examining the costs and benefits of inhibition in memory retrieval. Cognition 133, 358–370 (2014).
Ochsner, K. N., Silvers, J. A. & Buhle, J. T. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann. N. Y. Acad. Sci. 1251, E1–E24 (2012).
Powers, J. P. & LaBar, K. S. Regulating emotion through distancing: a taxonomy, neurocognitive model, and supporting meta-analysis. Neurosci. Biobehav. Rev. 96, 155–173 (2019).
Morawetz, C., Bode, S., Derntl, B. & Heekeren, H. R. The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: a meta-analysis of fMRI studies. Neurosci. Biobehav. Rev. 72, 111–128 (2017).
Frank, D. W. et al. Emotion regulation: quantitative meta-analysis of functional activation and deactivation. Neurosci. Biobehav. Rev. 45, 202–211 (2014).
Fullana, M. A. et al. Fear extinction in the human brain: a meta-analysis of fMRI studies in healthy participants. Neurosci. Biobehav. Rev. 88, 16–25 (2018).
Anderson, M. C. & Floresco, S. B. Prefrontal–hippocampal interactions supporting the extinction of emotional memories: the retrieval stopping model. Neuropsychopharmacology 47, 180–195 (2022). Introduces a model of fear extinction which argues that extinction operates in part, by spontaneously recruiting stopping, and reviews human and animal evidence consistent with this proposal.
Hunt, K. J., Knight, L. K. & Depue, B. E. Related neural networks underlie suppression of emotion, memory, motor processes as identified by data-driven analysis. BMC Neurosci. 24, 44 (2023).
Liu, W., Peeters, N., Fernández, G. & Kohn, N. Common neural and transcriptional correlates of inhibitory control underlie emotion regulation and memory control. Soc. Cogn. Affect. Neurosci. 15, 523–536 (2020).
Diesburg, D. A., Greenlee, J. D. & Wessel, J. R. Cortico-subcortical β burst dynamics underlying movement cancellation in humans. eLife 10, e70270 (2021).
Choo, Y., Matzke, D., Bowren, M. D. Jr, Tranel, D. & Wessel, J. R. Right inferior frontal gyrus damage is associated with impaired initiation of inhibitory control, but not its implementation. eLife 11, e79667 (2022).
Swann, N. et al. Intracranial EEG reveals a time- and frequency-specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. J. Neurosci. 29, 12675–12685 (2009).
Wessel, J. R., Conner, C. R., Aron, A. R. & Tandon, N. Chronometric electrical stimulation of right inferior frontal cortex increases motor braking. J. Neurosci. 33, 19611–19619 (2013).
Wagner, J., Wessel, J. R., Ghahremani, A. & Aron, A. R. Establishing a right frontal beta signature for stopping action in scalp EEG: implications for testing inhibitory control in other task contexts. J. Cogn. Neurosci. 30, 107–118 (2018).
Cole, M. W. et al. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat. Neurosci. 16, 1348–1355 (2013).
Boucher, L., Palmeri, T. J., Logan, G. D. & Schall, J. D. Inhibitory control in mind and brain: an interactive race model of countermanding saccades. Psychol. Rev. 114, 376–397 (2007).
Coxon, J. P., Stinear, C. M. & Byblow, W. D. Intracortical inhibition during volitional inhibition of prepared action. J. Neurophysiol. 95, 3371–3383 (2006).
Moscovitch, M., Cabeza, R., Winocur, G. & Nadel, L. Episodic memory and beyond: the hippocampus and neocortex in transformation. Annu. Rev. Psychol. 67, 105–134 (2016).
Rugg, M. D. & Vilberg, K. L. Brain networks underlying episodic memory retrieval. Curr. Opin. Neurobiol. 23, 255–260 (2013).
Hulbert, J. C., Henson, R. N. & Anderson, M. C. Inducing amnesia through systemic suppression. Nat. Commun. 7, 11003 (2016).
Yang, W. et al. Behavioral and neural correlates of memory suppression in subthreshold depression. Psychiatry Res. Neuroimaging 297, 111030 (2020).
Butler, A. J. & James, K. H. The neural correlates of attempting to suppress negative versus neutral memories. Cogn. Affect. Behav. Neurosci. 10, 182–194 (2010).
Buckner, R. L. & DiNicola, L. M. The brain’s default network: updated anatomy, physiology and evolving insights. Nat. Rev. Neurosci. 20, 593–608 (2019).
Ekstrom, A. How and when the fMRI BOLD signal relates to underlying neural activity: the danger in dissociation. Brain Res. Rev. 62, 233–244 (2010).
Epp, S. M. et al. Two distinct modes of hemodynamic responses in the human brain. Preprint at bioRxiv https://doi.org/10.1101/2023.12.08.570806 (2023).
Shmuel, A., Augath, M., Oeltermann, A. & Logothetis, N. K. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat. Neurosci. 9, 569–577 (2006).
Pasley, B. N., Inglis, B. A. & Freeman, R. D. Analysis of oxygen metabolism implies a neural origin for the negative BOLD response in human visual cortex. NeuroImage 36, 269–276 (2007).
Mullinger, K. J., Mayhew, S. D., Bagshaw, A. P., Bowtell, R. & Francis, S. T. Evidence that the negative BOLD response is neuronal in origin: a simultaneous EEG–BOLD–CBF study in humans. NeuroImage 94, 263–274 (2014).
Lauritzen, M., Mathiesen, C., Schaefer, K. & Thomsen, K. J. Neuronal inhibition and excitation, and the dichotomic control of brain hemodynamic and oxygen responses. NeuroImage 62, 1040–1050 (2012).
Boillat, Y., Xin, L., van der Zwaag, W. & Gruetter, R. Metabolite concentration changes associated with positive and negative BOLD responses in the human visual cortex: a functional MRS study at 7 tesla. J. Cereb. Blood Flow. Metab. 40, 488–500 (2020).
Rizio, A. A. & Dennis, N. A. The neural correlates of cognitive control: successful remembering and intentional forgetting. J. Cogn. Neurosci. 25, 297–312 (2013).
Fuentemilla, L. Memory: theta rhythm couples periodic reactivation during memory retrieval. Curr. Biol. 28, R1243–R1245 (2018).
Staresina, B. & Wimber, M. A neural chronometry of memory recall. Trends Cogn. Sci. 23, 1071–1085 (2019).
Herweg, N. A. et al. Theta-alpha oscillations bind the hippocampus, prefrontal cortex, and striatum during recollection: evidence from simultaneous EEG-fMRI. J. Neurosci. 36, 3579–3587 (2016).
Düzel, E. et al. A multivariate, spatiotemporal analysis of electromagnetic time–frequency data of recognition memory. NeuroImage 18, 185–197 (2003).
Osipova, D. et al. Theta and gamma oscillations predict encoding and retrieval of declarative memory. J. Neurosci. 26, 7523–7531 (2006).
Guderian, S. & Düzel, E. Induced theta oscillations mediate large-scale synchrony with mediotemporal areas during recollection in humans. Hippocampus 15, 901–912 (2005).
Fuentemilla, L., Barnes, G. R., Düzel, E. & Levine, B. Theta oscillations orchestrate medial temporal lobe and neocortex in remembering autobiographical memories. NeuroImage 85, 730–737 (2014).
Kota, S., Rugg, M. D. & Lega, B. C. Hippocampal theta oscillations support successful associative memory formation. J. Neurosci. 40, 9507–9518 (2020).
Herweg, N. A., Solomon, E. A. & Kahana, M. J. Theta oscillations in human memory. Trends Cogn. Sci. 24, 208–227 (2020).
Kam, J. W. Y. et al. Default network and frontoparietal control network theta connectivity supports internal attention. Nat. Hum. Behav. 3, 1263–1270 (2019).
Satish, A., Hellerstedt, R., Anderson, M. C. & Bergström, Z. M. EEG evidence that morally relevant autobiographical memories can be suppressed. Cogn. Affect. Behav. Neurosci. 22, 1290–1310 (2022).
Waldhauser, G. T., Bäuml, K. H. T. & Hanslmayr, S. Brain oscillations mediate successful suppression of unwanted memories. Cereb. Cortex 25, 4180–4190 (2015). Careful exploration of the brain oscillatory mechanisms of retrieval stopping, including evidence that suppression reduces medial-temporal lobe theta.
Kim, H. Dissociating the roles of the default-mode, dorsal, and ventral networks in episodic memory retrieval. NeuroImage 50, 1648–1657 (2010).
Grady, C. L. Meta-analytic and functional connectivity evidence from functional magnetic resonance imaging for an anterior to posterior gradient of function along the hippocampal axis. Hippocampus 30, 456–471 (2020).
Rugg, M. D. et al. Item memory, context memory and the hippocampus: fMRI evidence. Neuropsychologia 50, 3070–3079 (2012).
Drew, T. W., McCollough, A. W. & Vogel, E. K. Event-related potential measures of visual working memory. Clin. EEG Neurosci. 37, 286–291 (2006).
Chen, C. et al. Suppression of aversive memories associates with changes in early and late stages of neurocognitive processing. Neuropsychologia 50, 2839–2848 (2012).
Bergström, Z. M., Velmans, M., de Fockert, J. & Richardson-Klavehn, A. ERP evidence for successful voluntary avoidance of conscious recollection. Brain Res. 1151, 119–133 (2007).
Lin, X. et al. Observing the suppression of individual aversive memories from conscious awareness. Cereb. Cortex 34, bhae080 (2024).
Botvinick, M. M., Braver, T. S., Barch, D. M., Carter, C. S. & Cohen, J. D. Conflict monitoring and cognitive control. Psychol. Rev. 108, 624–652 (2001).
Botvinick, M. M. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn. Affect. Behav. Neurosci. 7, 356–366 (2007).
Alexander, W. H. & Brown, J. W. Hierarchical error representation: a computational model of anterior cingulate and dorsolateral prefrontal cortex. Neural Comput. 27, 2354–2410 (2015).
Vassena, E., Deraeve, J. & Alexander, W. H. Surprise, value and control in anterior cingulate cortex during speeded decision-making. Nat. Hum. Behav. 4, 412–422 (2020).
Cavanagh, J. F. & Frank, M. J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 18, 414–421 (2014).
Depue, B. E. et al. ERPs and neural oscillations during volitional suppression of memory retrieval. J. Cogn. Neurosci. 25, 1624–1633 (2013).
Kuhl, B. A., Dudukovic, N. M., Kahn, I. & Wagner, A. D. Decreased demands on cognitive control reveal the neural processing benefits of forgetting. Nat. Neurosci. 10, 908–914 (2007).
Leone, G. et al. Plasticity of human resilience mechanisms. Sci. Adv. 11, eadq8336 (2025).
Catarino, A., Küpper, C. S., Werner-Seidler, A., Dalgleish, T. & Anderson, M. C. Failing to forget: inhibitory-control deficits compromise memory suppression in posttraumatic stress disorder. Psychol. Sci. 26, 604–616 (2015).
Postel, C. et al. Variations in response to trauma and hippocampal subfield changes. Neurobiol. Stress 15, 100346 (2021). Detailed study of how the structural integrity of hippocampal subregions relates to deficits in intrusive memories of trauma and imaging markers of memory control ability in patients with post-traumatic stress disorder and traumatized controls.
Theves, S., Grande, X., Duzel, E. & Doeller, C. F. in The Oxford Handbook of Human Memory, Two Volume Pack (eds Kahana, M. J. & Wagner, A. D.) 988–1016 (Oxford Univ. Press, 2024).
Depue, B. E. A neuroanatomical model of prefrontal inhibitory modulation of memory retrieval. Neurosci. Biobehav. Rev. 36, 1382–1399 (2012).
Wessel, J. R. & Aron, A. R. On the globality of motor suppression: unexpected events and their influence on behavior and cognition. Neuron 93, 259–280 (2017).
Badry, R. et al. Suppression of human cortico-motoneuronal excitability during the stop-signal task. Clin. Neurophysiol. 120, 1717–1723 (2009).
Anderson, M. C. & Subbulakshmi, S. Amnesia in healthy people via hippocampal inhibition: a new forgetting mechanism. Q. J. Exp. Psychol. 77, 1–13 (2024).
Hulbert, J. C., Hirschstein, Z., Brontë, C. A. L. & Broughton, E. Unintended side effects of a spotless mind: theory and practice. Memory 26, 306–320 (2018).
Aggleton, J. P. & Brown, M. W. Episodic memory, amnesia, and the hippocampal–anterior thalamic axis. Behav. Brain Sci. 22, 425–444 (1999).
Eichenbaum, H., Sauvage, M., Fortin, N., Komorowski, R. & Lipton, P. Towards a functional organization of episodic memory in the medial temporal lobe. Neurosci. Biobehav. Rev. 36, 1597–1608 (2012).
Eichenbaum, H., Yonelinas, A. R. & Ranganath, C. The medial temporal lobe and recognition memory. Annu. Rev. Neurosci. 30, 123–152 (2007).
Zhu, Z., Anderson, M. C. & Wang, Y. Inducing forgetting of unwanted memories through subliminal reactivation. Nat. Commun. 13, 6496 (2022).
Zhu, Z. & Wang, Y. Forgetting unrelated episodic memories through suppression-induced amnesia. J. Exp. Psychol. Gen. 150, 401–413 (2021).
Duss, S. B., Oggier, S., Reber, T. P. & Henke, K. Formation of semantic associations between subliminally presented face-word pairs. Conscious. Cogn. 20, 928–935 (2011).
Henke, K. et al. Nonconscious formation and reactivation of semantic associations by way of the medial temporal lobe. Neuropsychologia 41, 863–876 (2003).
Reber, T. P., Luechinger, R., Boesiger, P. & Henke, K. Unconscious relational inference recruits the hippocampus. J. Neurosci. 32, 6138–6148 (2012).
Duss, S. B. et al. Unconscious relational encoding depends on hippocampus. Brain J. Neurol. 137, 3355–3370 (2014).
Degonda, N. et al. Implicit associative learning engages the hippocampus and interacts with explicit associative learning. Neuron 46, 505–520 (2005).
Miller, E. K. & Cohen, J. D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202 (2001).
Desimone, R. & Duncan, J. Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 18, 193–222 (1995).
Barbas, H. et al. Relationship of prefrontal connections to inhibitory systems in superior temporal areas in the rhesus monkey. Cereb. Cortex 15, 1356–1370 (2005).
Germuska, M., Saha, S., Fiala, J. & Barbas, H. Synaptic distinction of laminar-specific prefrontal–temporal pathways in primates. Cereb. Cortex 16, 865–875 (2006).
Medalla, M., Lera, P., Feinberg, M. & Barbas, H. Specificity in inhibitory systems associated with prefrontal pathways to temporal cortex in primates. Cereb. Cortex 17, i136–i150 (2007).
White, E. L. in Cortical Circuits: Synaptic Organization of the Cerebral Cortex Structure, Function, and Theory (ed. White, E. L.) 46–82 (Birkhäuser, 1989).
Grill-Spector, K., Kushnir, T., Hendler, T. & Malach, R. The dynamics of object-selective activation correlate with recognition performance in humans. Nat. Neurosci. 3, 837–843 (2000).
Bar, M. et al. Cortical mechanisms specific to explicit visual object recognition. Neuron 29, 529–535 (2001).
Henson, R. N. A. Neuroimaging studies of priming. Prog. Neurobiol. 70, 53–81 (2003).
Barron, H. C., Garvert, M. M. & Behrens, T. E. J. Repetition suppression: a means to index neural representations using BOLD? Philos. Trans. R. Soc. B Biol. Sci. 371, 20150355 (2016).
Hannula, D. E. & Ranganath, C. The eyes have it: hippocampal activity predicts expression of memory in eye movements. Neuron 63, 592–599 (2009).
Sheldon, S. A. M. & Moscovitch, M. Recollective performance advantages for implicit memory tasks. Memory 18, 681–697 (2010).
Wimmer, G. E. & Shohamy, D. Preference by association: how memory mechanisms in the hippocampus bias decisions. Science 338, 270–273 (2012).
Staresina, B. P. et al. Recollection in the human hippocampal–entorhinal cell circuitry. Nat. Commun. 10, 1503 (2019).
Danker, J. F. & Anderson, J. R. The ghosts of brain states past: remembering reactivates the brain regions engaged during encoding. Psychol. Bull. 136, 87–102 (2010).
McClelland, J. L., McNaughton, B. L. & O’Reilly, R. C. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 102, 419–457 (1995).
Hannula, D. E. & Greene, A. J. The hippocampus reevaluated in unconscious learning and memory: at a tipping point? Front. Hum. Neurosci. 6, 80 (2012).
Moscovitch, M. The hippocampus as a ‘stupid,’ domain-specific module: implications for theories of recent and remote memory, and of imagination. Can. J. Exp. Psychol. Rev. Can. Psychol. Exp. 62, 62–79 (2008).
Wimber, M., Maaß, A., Staudigl, T., Richardson-Klavehn, A. & Hanslmayr, S. Rapid memory reactivation revealed by oscillatory entrainment. Curr. Biol. 22, 1482–1486 (2012).
Waldhauser, G. T., Braun, V. & Hanslmayr, S. Episodic memory retrieval functionally relies on very rapid reactivation of sensory information. J. Neurosci. 36, 251–260 (2016).
Aggleton, J. P. A description of the amygdalo-hippocampal interconnections in the macaque monkey. Exp. Brain Res. 64, 515–526 (1986).
Saunders, R. C., Rosene, D. L. & Van Hoesen, G. W. Comparison of the efferents of the amygdala and the hippocampal formation in the rhesus monkey: II. Reciprocal and non-reciprocal connections. J. Comp. Neurol. 271, 185–207 (1988).
Suzuki, W. A. Neuroanatomy of the monkey entorhinal, perirhinal and parahippocampal cortices: organization of cortical inputs and interconnections with amygdala and striatum. Semin. Neurosci. 8, 3–12 (1996).
Witter, M. P., Groenewegen, H. J., Lopes da Silva, F. H. & Lohman, A. H. Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog. Neurobiol. 33, 161–253 (1989).
Mamat, Z. & Anderson, M. C. Improving mental health by training the suppression of unwanted thoughts. Sci. Adv. 9, eadh5292 (2023). An early experimental demonstration that training people to suppress upsetting thoughts of feared future events significantly improves mental health, contrary to widespread clinical belief.
Fawcett, J. M., Lawrence, M. A. & Taylor, T. L. The representational consequences of intentional forgetting: impairments to both the probability and fidelity of long-term memory. J. Exp. Psychol. Gen. 145, 56–81 (2016).
Yan, Y. et al. Reduced hippocampal–cortical connectivity during memory suppression predicts the ability to forget unwanted memories. Cereb. Cortex 33, 4189–4201 (2023).
Benoit, R. G., Davies, D. J. & Anderson, M. C. Reducing future fears by suppressing the brain mechanisms underlying episodic simulation. Proc. Natl Acad. Sci. USA 113, E8492–E8501 (2016). An important extension of the retrieval stopping model of memory control to the control of future fears, rather than memories of the past, relating this ability to anxiety.
Nishiyama, S. & Saito, S. Retrieval stopping can reduce distress from aversive memories. Cogn. Emot. 36, 957–974 (2022).
Corbetta, M. & Shulman, G. L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 3, 201–215 (2002).
Corbetta, M., Patel, G. & Shulman, G. L. The reorienting system of the human brain: from environment to theory of mind. Neuron 58, 306–324 (2008).
Yantis, S. & Jonides, J. Abrupt visual onsets and selective attention: evidence from visual search. J. Exp. Psychol. Hum. Percept. Perform. 10, 601–621 (1984).
Luck, S. J., Gaspelin, N., Folk, C. L., Remington, R. W. & Theeuwes, J. Progress toward resolving the attentional capture debate. Vis. Cogn. 29, 1–21 (2021).
Cabeza, R., Ciaramelli, E., Olson, I. R. & Moscovitch, M. The parietal cortex and episodic memory: an attentional account. Nat. Rev. Neurosci. 9, 613–625 (2008).
Cabeza, R., Ciaramelli, E. & Moscovitch, M. Cognitive contributions of the ventral parietal cortex: an integrative theoretical account. Trends Cogn. Sci. 16, 338–352 (2012).
Posner, M. I. Orienting of attention. Q. J. Exp. Psychol. 32, 3–25 (1980).
Anderson, M. C. & Spellman, B. A. On the status of inhibitory mechanisms in cognition: memory retrieval as a model case. Psychol. Rev. 102, 68–100 (1995).
Verschooren, S. & Egner, T. When the mind’s eye prevails: the internal dominance over external attention (IDEA) hypothesis. Psychon. Bull. Rev. 30, 1668–1688 (2023).
van Ede, F. & Nobre, A. C. Turning attention inside out: how working memory serves behavior. Annu. Rev. Psychol. 74, 137–165 (2023).
Cai, W., Ryali, S., Chen, T., Li, C.-S. R. & Menon, V. Dissociable roles of right inferior frontal cortex and anterior insula in inhibitory control: evidence from intrinsic and task-related functional parcellation, connectivity, and response profile analyses across multiple datasets. J. Neurosci. 34, 14652–14667 (2014).
Molnar-Szakacs, I. & Uddin, L. Q. Anterior insula as a gatekeeper of executive control. Neurosci. Biobehav. Rev. 139, 104736 (2022).
Menon, V. & D’Esposito, M. The role of PFC networks in cognitive control and executive function. Neuropsychopharmacology 47, 90–103 (2022).
Sridharan, D., Levitin, D. J. & Menon, V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl Acad. Sci. USA 105, 12569–12574 (2008).
Yang, W. et al. Memory suppression ability can be robustly predicted by the internetwork communication of frontoparietal control network. Cereb. Cortex 31, 3451–3461 (2021).
Shulman, G. L., Astafiev, S. V., McAvoy, M. P., d’Avossa, G. & Corbetta, M. Right TPJ deactivation during visual search: functional significance and support for a filter hypothesis. Cereb. Cortex 17, 2625–2633 (2007).
Solís‐Vivanco, R., Jensen, O. & Bonnefond, M. New insights on the ventral attention network: active suppression and involuntary recruitment during a bimodal task. Hum. Brain Mapp. 42, 1699–1713 (2020).
Todd, J. J., Fougnie, D. & Marois, R. Visual short-term memory load suppresses temporo-parietal junction activity and induces inattentional blindness. Psychol. Sci. 16, 965–972 (2005).
Malik, R., Li, Y., Schamiloglu, S. & Sohal, V. S. Top-down control of hippocampal signal-to-noise by prefrontal long-range inhibition. Cell 185, 1602–1617.e17 (2022).
Barbas, H., Ghashghaei, H., Dombrowski, S. M. & Rempel-Clower, N. L. Medial prefrontal cortices are unified by common connections with superior temporal cortices and distinguished by input from memory-related areas in the rhesus monkey. J. Comp. Neurol. 410, 343–367 (1999).
Ghashghaei, H. T., Hilgetag, C. C. & Barbas, H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. NeuroImage 34, 905–923 (2007).
Ongür, D., An, X. & Price, J. L. Prefrontal cortical projections to the hypothalamus in macaque monkeys. J. Comp. Neurol. 401, 480–505 (1998).
Rempel-Clower, N. L. & Barbas, H. Topographic organization of connections between the hypothalamus and prefrontal cortex in the rhesus monkey. J. Comp. Neurol. 398, 393–419 (1998).
Barbas, H. & Blatt, G. J. Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus 5, 511–533 (1995).
Cavada, C., Compañy, T., Tejedor, J., Cruz-Rizzolo, R. J. & Reinoso-Suárez, F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb. Cortex 10, 220–242 (2000).
Insausti, R. & Muñoz, M. Cortical projections of the non-entorhinal hippocampal formation in the cynomolgus monkey (Macaca fascicularis). Eur. J. Neurosci. 14, 435–451 (2001).
Rosene, D. L. & Van Hoesen, G. W. Hippocampal efferents reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science 198, 315–317 (1977).
Apergis-Schoute, J., Pinto, A. & Paré, D. Ultrastructural organization of medial prefrontal inputs to the rhinal cortices. Eur. J. Neurosci. 24, 135–144 (2006).
Bunce, J. G., Zikopoulos, B., Feinberg, M. & Barbas, H. Parallel prefrontal pathways reach distinct excitatory and inhibitory systems in memory-related rhinal cortices. J. Comp. Neurol. 521, 4260–4283 (2013).
Ludowig, E. et al. Active suppression in the mediotemporal lobe during directed forgetting. Neurobiol. Learn. Mem. 93, 352–361 (2010).
Barbas, H., Henion, T. H. & Dermon, C. R. Diverse thalamic projections to the prefrontal cortex in the rhesus monkey. J. Comp. Neurol. 313, 65–94 (1991).
Dermon, C. R. & Barbas, H. Contralateral thalamic projections predominantly reach transitional cortices in the rhesus monkey. J. Comp. Neurol. 344, 508–531 (1994).
Bertram, E. H. & Zhang, D. X. Thalamic excitation of hippocampal CA1 neurons: a comparison with the effects of CA3 stimulation. Neuroscience 92, 15–26 (1999).
Cassel, J.-C. et al. The reuniens and rhomboid nuclei: neuroanatomy, electrophysiological characteristics and behavioral implications. Prog. Neurobiol. 111, 34–52 (2013).
Dolleman-Van der Weel, M. J. & Witter, M. P. Nucleus reuniens thalami innervates gamma aminobutyric acid positive cells in hippocampal field CA1 of the rat. Neurosci. Lett. 278, 145–148 (2000).
Varela, C., Kumar, S., Yang, J. Y. & Wilson, M. A. Anatomical substrates for direct interactions between hippocampus, medial prefrontal cortex, and the thalamic nucleus reuniens. Brain Struct. Funct. 219, 911–929 (2014).
Vertes, R. P. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience 142, 1–20 (2006).
Vertes, R. P., Hoover, W. B., Szigeti-Buck, K. & Leranth, C. Nucleus reuniens of the midline thalamus: link between the medial prefrontal cortex and the hippocampus. Brain Res. Bull. 71, 601–609 (2007).
Andrianova, L. et al. Hippocampal CA1 pyramidal cells do not receive monosynaptic input from thalamic nucleus reuniens. Preprint at bioRxiv https://doi.org/10.1101/2021.09.30.462517 (2021).
Plas, S. L. et al. Neural circuits for the adaptive regulation of fear and extinction memory. Front. Behav. Neurosci. 18, 1352797 (2024).
Totty, M. S. et al. Thalamic nucleus reuniens coordinates prefrontal-hippocampal synchrony to suppress extinguished fear. Nat. Commun. 14, 6565 (2023).
Ratigan, H. C., Krishnan, S., Smith, S. & Sheffield, M. E. J. A thalamic-hippocampal CA1 signal for contextual fear memory suppression, extinction, and discrimination. Nat. Commun. 14, 6758 (2023).
Vertes, R. P., Hoover, W. B. & Viana Di Prisco, G. Theta rhythm of the hippocampus: subcortical control and functional significance. Behav. Cogn. Neurosci. Rev. 3, 173–200 (2004).
Freund, T. F. & Buzsáki, G. Interneurons of the hippocampus. Hippocampus 6, 347–470 (1996).
Hangya, B., Borhegyi, Z., Szilágyi, N., Freund, T. F. & Varga, V. GABAergic neurons of the medial septum lead the hippocampal network during theta activity. J. Neurosci. 29, 8094–8102 (2009).
Tzilivaki, A. et al. Hippocampal GABAergic interneurons and memory. Neuron 111, 3154–3175 (2023).
Hasselmo, M. E., Bodelón, C. & Wyble, B. P. A proposed function for hippocampal theta rhythm: separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput. 14, 793–817 (2002).
Mizumori, S. J., Perez, G. M., Alvarado, M. C., Barnes, C. A. & McNaughton, B. L. Reversible inactivation of the medial septum differentially affects two forms of learning in rats. Brain Res. 528, 12–20 (1990).
Butler, T. et al. Volume of the human septal forebrain region is a predictor of source memory accuracy. J. Int. Neuropsychol. Soc. 18, 157–161 (2012).
Tsanov, M. Basal forebrain impairment: understanding the mnemonic function of the septal region translates in therapeutic advances. Front. Neural Circuits 16, 916499 (2022).
Sans-Dublanc, A. et al. Septal GABAergic inputs to CA1 govern contextual memory retrieval. Sci. Adv. 6, eaba5003 (2020).
Tóth, K., Freund, T. F. & Miles, R. Disinhibition of rat hippocampal pyramidal cells by GABAergic afferents from the septum. J. Physiol. 500, 463–474 (1997).
Vertes, R. P. in Handbook of Behavioral Neuroscience (eds Müller, C. P. & Jacobs, B. L.) Vol. 21 277–292 (Elsevier, 2010).
Vertes, R. P., Fortin, W. J. & Crane, A. M. Projections of the median raphe nucleus in the rat. J. Comp. Neurol. 407, 555–582 (1999).
Schnider, A. & Ptak, R. Spontaneous confabulators fail to suppress currently irrelevant memory traces. Nat. Neurosci. 2, 677–681 (1999).
Schnider, A. Spontaneous confabulation and the adaptation of thought to ongoing reality. Nat. Rev. Neurosci. 4, 662–671 (2003).
Hasselmo, M. E., Schnell, E. & Barkai, E. Dynamics of learning and recall at excitatory recurrent synapses and cholinergic modulation in rat hippocampal region CA3. J. Neurosci. 15, 5249–5262 (1995).
Meeter, M., Murre, J. M. J. & Talamini, L. M. Mode shifting between storage and recall based on novelty detection in oscillating hippocampal circuits. Hippocampus 14, 722–741 (2004).
Zandbelt, B. B. & Vink, M. On the role of the striatum in response inhibition. PLoS ONE 5, e13848 (2010).
Zandbelt, B. B., Bloemendaal, M., Hoogendam, J. M., Kahn, R. S. & Vink, M. Transcranial magnetic stimulation and functional MRI reveal cortical and subcortical interactions during stop-signal response inhibition. J. Cogn. Neurosci. 25, 157–174 (2013).
Pas, P., Plessis, S. D., van den Munkhof, H. E., Gladwin, T. E. & Vink, M. Using subjective expectations to model the neural underpinnings of proactive inhibition. Eur. J. Neurosci. 49, 1575–1586 (2019).
Vink, M., Kaldewaij, R., Zandbelt, B. B., Pas, P. & du Plessis, S. The role of stop-signal probability and expectation in proactive inhibition. Eur. J. Neurosci. 41, 1086–1094 (2015).
Majid, D. S. A., Cai, W., Corey-Bloom, J. & Aron, A. R. Proactive selective response suppression is implemented via the basal ganglia. J. Neurosci. 33, 13259–13269 (2013).
Diesburg, D. A. & Wessel, J. R. The pause-then-cancel model of human action-stopping: theoretical considerations and empirical evidence. Neurosci. Biobehav. Rev. 129, 17–34 (2021).
Chatham, C. H. & Badre, D. Multiple gates on working memory. Curr. Opin. Behav. Sci. 1, 23–31 (2015).
Hirsch, C. R. & Mathews, A. A cognitive model of pathological worry. Behav. Res. Ther. 50, 636–646 (2012).
Watkins, E. R. & Roberts, H. Reflecting on rumination: consequences, causes, mechanisms and treatment of rumination. Behav. Res. Ther. 127, 103573 (2020).
Schacter, D. L., Benoit, R. G., De Brigard, F. & Szpunar, K. K. Episodic future thinking and episodic counterfactual thinking: intersections between memory and decisions. Neurobiol. Learn. Mem. 117, 14–21 (2015).
Benoit, R. G. & Schacter, D. L. Specifying the core network supporting episodic simulation and episodic memory by activation likelihood estimation. Neuropsychologia 75, 450–457 (2015).
Waldhauser, G. T. et al. The neural dynamics of deficient memory control in heavily traumatized refugees. Sci. Rep. 8, 13132 (2018).
Leone, G. et al. Altered predictive control during memory suppression in PTSD. Nat. Commun. 13, 3300 (2022).
Hertel, P. T. & Gerstle, M. Depressive deficits in forgetting. Psychol. Sci. 14, 573–578 (2003).
Noreen, S. & Ridout, N. Intentional forgetting in dysphoria: investigating the inhibitory effects of thought substitution using independent cues. J. Behav. Ther. Exp. Psychiatry 52, 110–118 (2016).
Zhang, D., Xie, H., Liu, Y. & Luo, Y. Neural correlates underlying impaired memory facilitation and suppression of negative material in depression. Sci. Rep. 6, 37556 (2016).
Marzi, T., Regina, A. & Righi, S. Emotions shape memory suppression in trait anxiety. Front. Psychol. 4, 1001 (2014).
Fawcett, J. M. et al. The origins of repetitive thought in rumination: separating cognitive style from deficits in inhibitory control over memory. J. Behav. Ther. Exp. Psychiatry 47, 1–8 (2015).
Quaedflieg, C. W. E. M., Schneider, T. R., Daume, J. & Engel, A. K. Stress impairs intentional memory control through altered theta oscillations in lateral parietal cortex. J. Neurosci. 40, 7739–7748 (2020).
Harrington, M. O. & Cairney, S. A. Sleep loss gives rise to intrusive thoughts. Trends Cogn. Sci. 25, 434–436 (2021).
Quaedflieg, C. W. E. M., Stoffregen, H. & Ashton, S. M. Cortisol reactivity impairs suppression-induced forgetting. Psychoneuroendocrinology 142, 105774 (2022).
Ashton, S. M., Benoit, R. G. & Quaedflieg, C. W. E. M. The impairing effect of acute stress on suppression-induced forgetting of future fears and its moderation by working memory capacity. Psychoneuroendocrinology 120, 104790 (2020).
Harrington, M. O. et al. Memory control deficits in the sleep-deprived human brain. Proc. Natl Acad. Sci. USA 122, e2400743122 (2025).
Caspi, A. & Moffitt, T. E. All for one and one for all: mental disorders in one dimension. Am. J. Psychiatry 175, 831–844 (2018).
Sevenster, D., Visser, R. M. & D’Hooge, R. A translational perspective on neural circuits of fear extinction: current promises and challenges. Neurobiol. Learn. Mem. 155, 113–126 (2018).
Fullana, M. A. et al. Human fear conditioning: from neuroscience to the clinic. Behav. Res. Ther. 124, 103528 (2020).
Singewald, N. & Holmes, A. Rodent models of impaired fear extinction. Psychopharmacology 236, 21–32 (2019).
Craske, M. G., Hermans, D. & Vervliet, B. State-of-the-art and future directions for extinction as a translational model for fear and anxiety. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373, 20170025 (2018).
Dunsmoor, J. E., Cisler, J. M., Fonzo, G. A., Creech, S. K. & Nemeroff, C. B. Laboratory models of post-traumatic stress disorder: the elusive bridge to translation. Neuron 110, 1754–1776 (2022).
Wang, Y., Zhu, Z., Hu, J., Schiller, D. & Li, J. Active suppression prevents the return of threat memory in humans. Commun. Biol. 4, 609 (2021). An early demonstration that retrieval suppression might augment fear extinction, improving its durability and generalizability.
Kalisch, R., Russo, S. J. & Müller, M. B. Neurobiology and systems biology of stress resilience. Physiol. Rev. 104, 1205–1263 (2024).
Hulbert, J. C. & Anderson, M. C. What doesn’t kill you makes you stronger: psychological trauma and its relationship to enhanced memory control. J. Exp. Psychol. Gen. 147, 1931–1949 (2018).
Berry, J. A., Guhle, D. C. & Davis, R. L. Active forgetting and neuropsychiatric diseases. Mol. Psychiatry 29, 2810–2820 (2024).
Burback, L., Brult-Phillips, S., Nijdam, M. J., McFarlane, A. & Vermetten, E. Treatment of posttraumatic stress disorder: a state-of-the-art review. Curr. Neuropharmacol. 22, 557–635 (2024).
Miyake, A. et al. The unity and diversity of executive functions and their contributions to complex ‘frontal lobe’ tasks: a latent variable analysis. Cogn. Psychol. 41, 49–100 (2000).
Brewin, C. R. The nature and significance of memory disturbance in posttraumatic stress disorder. Annu. Rev. Clin. Psychol. 7, 203–227 (2011). Synthesis of evidence for memory deficits in post-traumatic stress disorder and their implications.
Guez, J. et al. Traumatic stress is linked to a deficit in associative episodic memory. J. Trauma. Stress 24, 260–267 (2011).
Guez, J. et al. Associative memory impairment in acute stress disorder: characteristics and time course. Psychiatry Res. 209, 479–484 (2013).
Kopelman, M. D. Anomalies of autobiographical memory. J. Int. Neuropsychol. Soc. 25, 1061–1075 (2019).
Marsh, L. C. et al. Prefrontally mediated inhibition of memory systems in dissociative amnesia. Psychol. Med. 54, 1–9 (2025).
Anderson, M. C. & Huddleston, E. in True and False Recovered Memories: Toward a Reconciliation of the Debate (ed. Belli, R. F.) 53–120 (Springer, 2012).
Nørby, S. Mnemonic emotion regulation: a three-process model. Cogn. Emot. 33, 959–975 (2019).
Fawcett, J. M. & Hulbert, J. C. The many faces of forgetting: toward a constructive view of forgetting in everyday life. J. Appl. Res. Mem. Cogn. 9, 1–18 (2020).
Nørby, S. Forgetting and emotion regulation in mental health, anxiety and depression. Memory 26, 342–363 (2018).
Satish, A., Hellerstedt, R., Anderson, M. C. & Bergström, Z. M. Memory control immediately improves unpleasant emotions associated with autobiographical memories of past immoral actions. Cogn. Emot. 38, 1032–1047 (2024).
Chalkia, A., Vanhasbroeck, N., Van Oudenhove, L., Kindt, M. & Beckers, T. Emotional associative memory is disrupted by directed forgetting. Commun. Psychol. 1, 24 (2023).
Hayes-Skelton, S. A. & Eustis, E. H. in Clinical Handbook of Fear and Anxiety: Maintenance Processes and Treatment Mechanisms (eds Abramowitz, J. S. & Blakey, S. M.) 115–131 (American Psychological Association, 2020).
Wegner, D. M. Ironic processes of mental control. Psychol. Rev. 101, 34–52 (1994).
Wegner, D. M. How to think, say, or do precisely the worst thing for any occasion. Science 325, 48–50 (2009).
Pelkey, K. A. et al. Hippocampal GABAergic inhibitory interneurons. Physiol. Rev. 97, 1619–1747 (2017).
Heckers, S. & Konradi, C. GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophr. Res. 167, 4–11 (2015). Reviews diverse evidence for the role of hippocampal GABAergic in schizophrenia, illustrating how deficient control over hippocampal activity can promote intrusive symptoms.
Osuch, E. A. et al. Regional cerebral blood flow correlated with flashback intensity in patients with posttraumatic stress disorder. Biol. Psychiatry 50, 246–253 (2001).
Patel, R., Spreng, R. N., Shin, L. M. & Girard, T. A. Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 36, 2130–2142 (2012).
Tregellas, J. R. et al. Intrinsic hippocampal activity as a biomarker for cognition and symptoms in schizophrenia. Am. J. Psychiatry 171, 549–556 (2014).
Konradi, C. et al. Hippocampal interneurons are abnormal in schizophrenia. Schizophr. Res. 131, 165–173 (2011).
Whitfield-Gabrieli, S. & Ford, J. M. Default mode network activity and connectivity in psychopathology. Annu. Rev. Clin. Psychol. 8, 49–76 (2012). Influential review documenting the prevalence of default network hyperactivity in mental illness, consistent with the current proposal that inhibitory regulation of hippocampal activity helps control intrusive symptomatology.
Sheline, Y. I. et al. The default mode network and self-referential processes in depression. Proc. Natl Acad. Sci. USA 106, 1942–1947 (2009).
Lieberman, J. A. et al. Hippocampal dysfunction in the pathophysiology of schizophrenia: a selective review and hypothesis for early detection and intervention. Mol. Psychiatry 23, 1764–1772 (2018).
Roeske, M. J., Konradi, C., Heckers, S. & Lewis, A. S. Hippocampal volume and hippocampal neuron density, number and size in schizophrenia: a systematic review and meta-analysis of postmortem studies. Mol. Psychiatry 26, 3524–3535 (2021).
Gilani, A. I. et al. Interneuron precursor transplants in adult hippocampus reverse psychosis-relevant features in a mouse model of hippocampal disinhibition. Proc. Natl Acad. Sci. USA 111, 7450–7455 (2014).
Schobel, S. A. et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron 78, 81–93 (2013).
Rayner, G., Jackson, G. & Wilson, S. Cognition-related brain networks underpin the symptoms of unipolar depression: evidence from a systematic review. Neurosci. Biobehav. Rev. 61, 53–65 (2016).
Hu, W., Zhang, M., Czéh, B., Flügge, G. & Zhang, W. Stress impairs GABAergic network function in the hippocampus by activating nongenomic glucocorticoid receptors and affecting the integrity of the parvalbumin-expressing neuronal network. Neuropsychopharmacol 35, 1693–1707 (2010).
Chen, S. et al. Defects of parvalbumin-positive interneurons in the ventral dentate gyrus region are implicated depression-like behavior in mice. Brain Behav. Immun. 99, 27–42 (2022).
Perez-Rando, M. et al. Impact of stress on inhibitory neuronal circuits, our tribute to Bruce McEwen. Neurobiol. Stress 19, 100460 (2022).
Crestani, F. et al. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nat. Neurosci. 2, 833–839 (1999).
Crestani, F. et al. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc. Natl Acad. Sci. USA 99, 8980–8985 (2002).
Yee, B. K. et al. GABA receptors containing the alpha5 subunit mediate the trace effect in aversive and appetitive conditioning and extinction of conditioned fear. Eur. J. Neurosci. 20, 1928–1936 (2004).
Hatoum, A. S. et al. Genome-wide association study shows that executive functioning is influenced by GABAergic processes and is a neurocognitive genetic correlate of psychiatric disorders. Biol. Psychiatry 93, 59–70 (2023).
Christoff, K., Irving, Z. C., Fox, K. C. R., Spreng, R. N. & Andrews-Hanna, J. R. Mind-wandering as spontaneous thought: a dynamic framework. Nat. Rev. Neurosci. 17, 718–731 (2016).
Kucyi, A., Kam, J. W. Y., Andrews-Hanna, J. R., Christoff, K. & Whitfield-Gabrieli, S. Recent advances in the neuroscience of spontaneous and off-task thought: implications for mental health. Nat. Ment. Health 1, 827–840 (2023).
Smallwood, J. & Schooler, J. W. The science of mind wandering: empirically navigating the stream of consciousness. Annu. Rev. Psychol. 66, 487–518 (2015).
Xia, C. H. et al. Linked dimensions of psychopathology and connectivity in functional brain networks. Nat. Commun. 9, 3003 (2018).
Acknowledgements
This work was supported by Medical Research Council grant MC-A060-5PR00 (M.C.A.).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, contributed substantially to discussion of the content and reviewed and/or edited the manuscript before submission. M.C.A. wrote the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neuroscience thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Default mode network
-
(DMN). A group of functionally connected brain regions (including the hippocampus) that are more active when the brain is in a resting state, not focused on external tasks, and engaged in internally oriented thoughts.
- Effective connectivity
-
An estimate of the influence that one brain region exerts over another, often assessed with model-based connectivity approaches such as dynamic causal modelling to determine the direction and strength of causal interactions between brain regions.
- Fear extinction
-
A learning process through which a conditioned fear response diminishes after repeated exposure to the conditioned stimulus without the aversive outcome.
- Functional connectivity
-
The level of statistical dependence over time (for example, correlations) of the activity of different brain regions, putatively reflecting the degree to which those regions may be communicating and working together in coordinated fashion.
- Inhibitory control
-
A core cognitive control function that enables organisms to suppress dominant motor, cognitive or affective responses to stimuli if circumstances or goals require those responses to be stopped.
- Intrusive thoughts
-
Involuntarily retrieved ideas, images or memories that interrupt ongoing thought and that are often distressing and repeated.
- Multiple-demand control network
-
A group of brain regions, primarily in the frontal and parietal cortex, that are activated across a wide range of cognitively demanding tasks, suggesting a role in flexible cognitive control and integration.
- Pattern completion
-
A key mechanism of memory retrieval, supported by the hippocampus, which allows an organism to retrieve a full event, by reinstating the full pattern of activity across neurons representing the event, when a subset of those neurons are activated by cue input.
- Representations
-
A pattern of neural activity that encodes information about stimuli, events, concepts or actions, allowing the brain to interpret, store and manipulate information to guide perception, thought and behaviour.
- Stop-signal action-stopping task
-
A task that quantifies motor response inhibition speed by having participants respond as quickly as possible to ‘go’ stimuli and then, on a small fraction of trials, withhold that response when a ‘stop’ signal tone occurs. By progressively increasing the delay after motor preparation begins at which the stop tone occurs, the challenge of stopping the response increases, and an estimate of the speed of the underlying stopping process can be derived.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Anderson, M.C., Crespo-Garcia, M. & Subbulakshmi, S. Brain mechanisms underlying the inhibitory control of thought. Nat. Rev. Neurosci. 26, 415–437 (2025). https://doi.org/10.1038/s41583-025-00929-y
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41583-025-00929-y