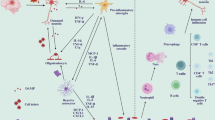
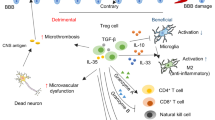
Abstract
Stroke remains a leading cause of disability owing to the irreversible neuronal loss that it causes and the limited regenerative capacity of the CNS. Although reperfusion therapies such as thrombolysis and mechanical thrombectomy can restore blood flow after stroke, their stringent eligibility criteria leave many patients without treatment options. The immune response, involving complex interactions between brain-resident and peripheral immune cells, has a critical role in stroke recovery. Stem cell-based therapies, particularly those involving neural stem cells and mesenchymal stem cells, may be able to reshape the inflammatory microenvironment after stroke, mitigating secondary injury and promoting tissue repair. However, the precise mechanisms driving their effects remain incompletely understood, hindering clinical translation. In this Review, we highlight the bidirectional crosstalk between stem cells and immune cells (including microglia, T cells and peripheral immune cells) and discuss how these interactions influence neuroinflammation, neural plasticity and circuit remodelling in stroke recovery. We examine key determinants of stem cell therapy efficacy, emphasizing the role of stem cell–immune cell interactions, and discuss targeted strategies to enhance immune modulation and neuroprotection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Tsao, C. W. et al. Heart disease and stroke statistics — 2023 update: a report from the American Heart Association. Circulation 147, e93–e621 (2023).
Iadecola, C., Buckwalter, M. S. & Anrather, J. Immune responses to stroke: mechanisms, modulation, and therapeutic potential. J. Clin. Invest. 130, 2777–2788 (2020).
Man, S. et al. Trends in stroke thrombolysis care metrics and outcomes by race and ethnicity, 2003–2021. JAMA Netw. Open 7, e2352927 (2024).
Kamel, H. et al. Access to mechanical thrombectomy for ischemic stroke in the United States. Stroke 52, 2554–2561 (2021).
Ganesh, A. et al. From three-months to five-years: sustaining long-term benefits of endovascular therapy for ischemic stroke. Front. Neurol. 12, 2021 (2021).
Rajkovic, O., Potjewyd, G. & Pinteaux, E. Regenerative medicine therapies for targeting neuroinflammation after stroke. Front. Neurol. 9, 734 (2018).
Duan, M., Xu, Y., Li, Y., Feng, H. & Chen, Y. Targeting brain-peripheral immune responses for secondary brain injury after ischemic and hemorrhagic stroke. J. Neuroinflammation 21, 102 (2024).
Liesz, A. et al. FTY720 reduces post-ischemic brain lymphocyte influx but does not improve outcome in permanent murine cerebral ischemia. PLoS ONE 6, e21312 (2011).
Qin, C. et al. Signaling pathways involved in ischemic stroke: molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 7, 215 (2022).
Fu, Y., Liu, Q., Anrather, J. & Shi, F. D. Immune interventions in stroke. Nat. Rev. Neurol. 11, 524–535 (2015).
Tan, N., Xin, W., Huang, M. & Mao, Y. Mesenchymal stem cell therapy for ischemic stroke: novel insight into the crosstalk with immune cells. Front. Neurol. 13, 1048113 (2022).
Nie, L. et al. Directional induction of neural stem cells, a new therapy for neurodegenerative diseases and ischemic stroke. Cell Death Discov. 9, 215 (2023).
Satani, N. et al. World-wide efficacy of bone marrow derived mesenchymal stromal cells in preclinical ischemic stroke models: systematic review and meta-analysis. Front. Neurol. 10, 405 (2019).
Baker, E. W., Kinder, H. A. & West, F. D. Neural stem cell therapy for stroke: a multimechanistic approach to restoring neurological function. Brain Behav. 9, e01214 (2019).
Anthony, S., Cabantan, D., Monsour, M. & Borlongan, C. V. Neuroinflammation, stem cells, and stroke. Stroke 53, 1460–1472 (2022).
Martino, G. & Pluchino, S. The therapeutic potential of neural stem cells. Nat. Rev. Neurosci. 7, 395–406 (2006).
Eckert, A. et al. Bystander effect fuels human induced pluripotent stem cell-derived neural stem cells to quickly attenuate early stage neurological deficits after stroke. Stem Cell Transl. Med. 4, 841–851 (2015).
Crosio, C., Valle, C., Casciati, A., Iaccarino, C. & Carrì, M. T. Astroglial inhibition of NF-κB does not ameliorate disease onset and progression in a mouse model for amyotrophic lateral sclerosis (ALS). PLoS ONE 6, e17187 (2011).
Zeng, J. et al. The mechanism of microglia-mediated immune inflammation in ischemic stroke and the role of natural botanical components in regulating microglia: a review. Front. Immunol. 13, 1047550 (2022).
Fan, P.-l, Wang, S.-s, Chu, S.-F & Chen, N.-H. Time-dependent dual effect of microglia in ischemic stroke. Neurochem. Int. 169, 105584 (2023).
D’Mello, C., Le, T. & Swain, M. G. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J. Neurosci. 29, 2089–2102 (2009).
Chiarini, A., Gui, L., Viviani, C., Armato, U. & Dal Prà, I. NLRP3 inflammasome’s activation in acute and chronic brain diseases — an update on pathogenetic mechanisms and therapeutic perspectives with respect to other inflammasomes. Biomedicines 11, 999 (2023).
Ma, Y., Liu, Y., Zhang, Z. & Yang, G. Y. Significance of complement system in ischemic stroke: a comprehensive review. Aging Dis. 10, 429–462 (2019).
Justicia, C. et al. Neutrophil infiltration increases matrix metalloproteinase-9 in the ischemic brain after occlusion/reperfusion of the middle cerebral artery in rats. J. Cereb. Blood Flow Metab. 23, 1430–1440 (2003).
Han, D., Liu, H. & Gao, Y. The role of peripheral monocytes and macrophages in ischemic stroke. Neurol. Sci. 41, 3589–3607 (2020).
Li, L. et al. The specific role of reactive astrocytes in stroke. Front. Cell Neurosci. 16, 850866 (2022).
Llovera, G. et al. The choroid plexus is a key cerebral invasion route for T cells after stroke. Acta Neuropathol. 134, 851–868 (2017).
Felger, J. C. et al. Brain dendritic cells in ischemic stroke: time course, activation state, and origin. Brain Behav. Immun. 24, 724–737 (2010).
Jin, W. N. et al. Brain ischemia induces diversified neuroantigen-specific T-cell responses that exacerbate brain injury. Stroke 49, 1471–1478 (2018).
Fassbender, K., Bertsch, T., Mielke, O., Mühlhauser, F. & Hennerici, M. Adhesion molecules in cerebrovascular diseases. Stroke 30, 1647–1650 (1999).
Yan, J. et al. Frequency and function of regulatory T cells after ischaemic stroke in humans. J. Neuroimmunol. 243, 89–94 (2012).
Liesz, A. et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat. Med. 15, 192–199 (2009).
Lee, Y. et al. Therapeutically targeting neuroinflammation and microglia after acute ischemic stroke. Biomed. Res. Int. 2014, 297241 (2014).
Monsour, M. & Borlongan, C. V. The central role of peripheral inflammation in ischemic stroke. J. Cereb. Blood Flow Metab. 43, 622–641 (2023).
Xie, L., He, M., Ying, C. & Chu, H. Mechanisms of inflammation after ischemic stroke in brain-peripheral crosstalk. Front. Mol. Neurosci. 17, 1400808 (2024).
Ziebell, J. M. & Morganti-Kossmann, M. C. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics 7, 22–30 (2010).
Li, Y. & Zhang, J. Animal models of stroke. Anim. Model. Exp. Med. 4, 204–219 (2021).
GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 20, 795–820 (2021).
Oh, J., Lee, Y. D. & Wagers, A. J. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat. Med. 20, 870–880 (2014).
Finger, C. E., Moreno-Gonzalez, I., Gutierrez, A., Moruno-Manchon, J. F. & McCullough, L. D. Age-related immune alterations and cerebrovascular inflammation. Mol. Psychiatry 27, 803–818 (2022).
Shi, L. et al. Genome-wide transcriptomic analysis of microglia reveals impaired responses in aged mice after cerebral ischemia. J. Cereb. Blood Flow Metab. 40, S49–s66 (2020).
Wlodarek, L., Alibhai, F. J., Wu, J., Li, S. H. & Li, R. K. Stroke-induced neurological dysfunction in aged mice is attenuated by preconditioning with young Sca-1+ stem cells. Stem Cell 40, 564–576 (2022).
Khamaisi, M. & Balanson, S. E. Stem cells for diabetes complications: a future potential cure. Rambam Maimonides Med. J. 8, e0008 (2017).
Xu, J. & Zuo, C. The fate status of stem cells in diabetes and its role in the occurrence of diabetic complications. Front. Mol. Biosci. 8, 745035 (2021).
Khullar, M., Mittal, A. & Randhawa, H. K. in Hypertension — An Update (ed. M. Khullar) (IntechOpen, 2022).
Lombardo, M. F. et al. Effects of aging on endothelial progenitor cells (EPCs) subpopulations in peripheral blood: a possible rationale for age-associated vascular dysfunction. BMC Geriatr. 10, A110 (2010).
Pabón, M. M., Ji, X. M., Fernandez, J. W. & Borlongan, C. V. Gender-linked stem cell alterations in stroke and postpartum depression. CNS Neurosci. Ther. 21, 348–356 (2015).
Heslop, J. A. et al. Concise review: workshop review: understanding and assessing the risks of stem cell-based therapies. Stem Cell Transl. Med. 4, 389–400 (2015).
Tang, Y., Yu, P. & Cheng, L. Current progress in the derivation and therapeutic application of neural stem cells. Cell Death Dis. 8, e3108–e3108 (2017).
Li, Y. et al. Unveiling the immunogenicity of allogeneic mesenchymal stromal cells: challenges and strategies for enhanced therapeutic efficacy. Biomed. Pharmacother. 180, 117537 (2024).
Hernández, R. et al. Differentiation of human mesenchymal stem cells towards neuronal lineage: clinical trials in nervous system disorders. Biomol. Ther. 28, 34–44 (2020).
Krueger, T. E. G., Thorek, D. L. J., Denmeade, S. R., Isaacs, J. T. & Brennen, W. N. Concise review: mesenchymal stem cell-based drug delivery: the good, the bad, the ugly, and the promise. Stem Cells Transl. Med. 7, 651–663 (2018).
Pluchino, S., Smith, J. A. & Peruzzotti-Jametti, L. Promises and limitations of neural stem cell therapies for progressive multiple sclerosis. Trends Mol. Med. 26, 898–912 (2020).
Tuazon, J. P. et al. Neural stem cells. Adv. Exp. Med. Biol. 1201, 79–91 (2019).
Toma, C., Wagner, W. R., Bowry, S., Schwartz, A. & Villanueva, F. Fate of culture-expanded mesenchymal stem cells in the microvasculature: in vivo observations of cell kinetics. Circ. Res. 104, 398–402 (2009).
Okano, H. et al. Steps toward safe cell therapy using induced pluripotent stem cells. Circ. Res. 112, 523–533 (2013).
Herberts, C. A., Kwa, M. S. G. & Hermsen, H. P. H. Risk factors in the development of stem cell therapy. J. Transl. Med. 9, 29 (2011).
Sun, L. et al. Co-transplantation of human umbilical cord mesenchymal stem cells and human neural stem cells improves the outcome in rats with spinal cord injury. Cell Transplant. 28, 893–906 (2019).
Kaminska, A., Radoszkiewicz, K., Rybkowska, P., Wedzinska, A. & Sarnowska, A. Interaction of neural stem cells (NSCs) and mesenchymal stem cells (MSCs) as a promising approach in brain study and nerve regeneration. Cells 11, 1464 (2022).
Hosseini, S. M. et al. Combination cell therapy with mesenchymal stem cells and neural stem cells for brain stroke in rats. Int. J. Stem Cell 8, 99–105 (2015).
Urrutia, D. N. et al. Comparative study of the neural differentiation capacity of mesenchymal stromal cells from different tissue sources: an approach for their use in neural regeneration therapies. PLoS ONE 14, e0213032 (2019).
Qiao, L.-Y. et al. A two-year follow-up study of cotransplantation with neural stem/progenitor cells and mesenchymal stromal cells in ischemic stroke patients. Cell Transplant. 23, 65–72 (2014).
Wang, L. et al. IFN-γ and TNF-α synergistically induce mesenchymal stem cell impairment and tumorigenesis via NFκB signaling. Stem Cell 31, 1383–1395 (2013).
Hess, D. C. et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 16, 360–368 (2017).
Houkin, K. et al. Allogeneic stem cell therapy for acute ischemic stroke: the phase 2/3 TREASURE randomized clinical trial. JAMA Neurol. 81, 154–162 (2024).
Wang, L. Q. et al. Timing and dose regimens of marrow mesenchymal stem cell transplantation affect the outcomes and neuroinflammatory response after ischemic stroke. CNS Neurosci. Ther. 20, 317–326 (2014).
Doeppner, T. R. et al. Effects of acute versus post-acute systemic delivery of neural progenitor cells on neurological recovery and brain remodeling after focal cerebral ischemia in mice. Cell Death Dis. 5, e1386 (2014).
Yavagal, D. R. et al. Efficacy and dose-dependent safety of intra-arterial delivery of mesenchymal stem cells in a rodent stroke model. PLoS ONE 9, e93735 (2014).
Yang, M. et al. Changes in host blood factors and brain glia accompanying the functional recovery after systemic administration of bone marrow stem cells in ischemic stroke rats. Cell Transpl. 19, 1073–1084 (2010).
Mine, Y. et al. Grafted human neural stem cells enhance several steps of endogenous neurogenesis and improve behavioral recovery after middle cerebral artery occlusion in rats. Neurobiol. Dis. 52, 191–203 (2013).
Darsalia, V. et al. Cell number and timing of transplantation determine survival of human neural stem cell grafts in stroke-damaged rat brain. J. Cereb. Blood Flow Metab. 31, 235–242 (2011).
Chen, L., Zhang, G., Gu, Y. & Guo, X. Meta-analysis and systematic review of neural stem cells therapy for experimental ischemia stroke in preclinical studies. Sci. Rep. 6, 32291 (2016).
Hill, W. D. et al. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J. Neuropathol. Exp. Neurol. 63, 84–96 (2004).
Abe, K. Therapeutic potential of neurotrophic factors and neural stem cells against ischemic brain injury. J. Cereb. Blood Flow Metab. 20, 1393–1408 (2000).
Jiao, Y., Liu, Y. W., Chen, W. G. & Liu, J. Neuroregeneration and functional recovery after stroke: advancing neural stem cell therapy toward clinical application. Neural Regen. Res. 16, 80–92 (2021).
Rodríguez-Frutos, B. et al. Stem cell therapy and administration routes after stroke. Transl. Stroke Res. 7, 378–387 (2016).
Li, Y., Chen, J., Wang, L., Lu, M. & Chopp, M. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology 56, 1666–1672 (2001).
Kamiya, N. et al. Intra-arterial transplantation of bone marrow mononuclear cells immediately after reperfusion decreases brain injury after focal ischemia in rats. Life Sci. 83, 433–437 (2008).
Misra, V., Ritchie, M. M., Stone, L. L., Low, W. C. & Janardhan, V. Stem cell therapy in ischemic stroke: role of IV and intra-arterial therapy. Neurology 79, S207–S212 (2012).
Lee, S. T. et al. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain 131, 616–629 (2008).
Doeppner, T. R. et al. Post-stroke transplantation of adult subventricular zone derived neural progenitor cells — a comprehensive analysis of cell delivery routes and their underlying mechanisms. Exp. Neurol. 273, 45–56 (2015).
Zhang, H.-l et al. Comparisons of the therapeutic effects of three different routes of bone marrow mesenchymal stem cell transplantation in cerebral ischemic rats. Brain Res. 1680, 143–154 (2018).
Gutiérrez-Fernández, M. et al. Functional recovery after hematic administration of allogenic mesenchymal stem cells in acute ischemic stroke in rats. Neuroscience 175, 394–405 (2011).
Jin, K. et al. Comparison of ischemia-directed migration of neural precursor cells after intrastriatal, intraventricular, or intravenous transplantation in the rat. Neurobiol. Dis. 18, 366–374 (2005).
Savitz, S. I., Dinsmore, J. H., Wechsler, L. R., Rosenbaum, D. M. & Caplan, L. R. Cell therapy for stroke. Neurotherapeutics 1, 406–414 (2004).
Li, L. et al. Effects of administration route on migration and distribution of neural progenitor cells transplanted into rats with focal cerebral ischemia, an MRI study. J. Cereb. Blood Flow Metab. 30, 653–662 (2010).
Walczak, P. et al. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke 39, 1569–1574 (2008).
Chua, J. Y. et al. Intra-arterial injection of neural stem cells using a microneedle technique does not cause microembolic strokes. J. Cereb. Blood Flow Metab. 31, 1263–1271 (2011).
Burgess, A. et al. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood–brain barrier. PLoS ONE 6, e27877 (2011).
Cheng, Y. et al. Intravenously delivered neural stem cells migrate into ischemic brain, differentiate and improve functional recovery after transient ischemic stroke in adult rats. Int. J. Clin. Exp. Pathol. 8, 2928–2936 (2015).
Yoo, S. W. et al. Immune following suppression mesenchymal stem cell transplantation in the ischemic brain is mediated by TGF-β. Neurobiol. Dis. 58, 249–257 (2013).
Cheng, Q. et al. Human umbilical cord mesenchymal stem cells protect against ischemic brain injury in mouse by regulating peripheral immunoinflammation. Brain Res. 1594, 293–304 (2015).
Łukowicz, K., Grygier, B. & Basta-Kaim, A. Emerging role of neural stem/progenitor cell secretome in brain inflammatory response modulation. Pharmacol. Rep. 77, 907–920 (2025).
Wang, Y. et al. Mechanism of inflammatory response and therapeutic effects of stem cells in ischemic stroke: current evidence and future perspectives. Neural Regen. Res. 20, 67–81 (2025).
Zhu, P. et al. Potential mechanism and perspectives of mesenchymal stem cell therapy for ischemic stroke: a review. Glob. Med. Genet. 11, 278–284 (2024).
Sheikh, A. M. et al. Mesenchymal stem cell transplantation modulates neuroinflammation in focal cerebral ischemia: contribution of fractalkine and IL-5. Neurobiol. Dis. 41, 717–724 (2011).
Liu, Y. et al. Mesenchymal stem cells inhibit lipopolysaccharide-induced inflammatory responses of BV2 microglial cells through TSG-6. J. Neuroinflammation 11, 135 (2014).
Schäfer, R. et al. Modulating endothelial adhesion and migration impacts stem cell therapies efficacy. EBioMedicine 60, 102987 (2020).
Huang, L., Wong, S., Snyder, E. Y., Hamblin, M. H. & Lee, J. P. Human neural stem cells rapidly ameliorate symptomatic inflammation in early-stage ischemic-reperfusion cerebral injury. Stem Cell Res. Ther. 5, 129 (2014).
Wang, X. et al. SDF-1 secreted by mesenchymal stem cells promotes the migration of endothelial progenitor cells via CXCR4/PI3K/AKT pathway. J. Mol. Histol. 52, 1155–1164 (2021).
Liu, Y. et al. AMSC-derived exosomes alleviate lipopolysaccharide/d-galactosamine-induced acute liver failure by miR-17-mediated reduction of TXNIP/NLRP3 inflammasome activation in macrophages. EBioMedicine 36, 140–150 (2018).
Capone, C. et al. Neurosphere-derived cells exert a neuroprotective action by changing the ischemic microenvironment. PLoS ONE 2, e373 (2007).
Lu, P., Jones, L. L., Snyder, E. Y. & Tuszynski, M. H. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp. Neurol. 181, 115–129 (2003).
Li, X. et al. Intravenously delivered allogeneic mesenchymal stem cells bidirectionally regulate inflammation and induce neurotrophic effects in distal middle cerebral artery occlusion rats within the first 7 days after stroke. Cell Physiol. Biochem. 46, 1951–1970 (2018).
Yoshida, Y. et al. Intravenous administration of human amniotic mesenchymal stem cells in the subacute phase of cerebral infarction in a mouse model ameliorates neurological disturbance by suppressing blood brain barrier disruption and apoptosis via immunomodulation. Cell Transpl. 30, 9636897211024183 (2021).
Tobin, M. K. et al. Activated mesenchymal stem cells induce recovery following stroke via regulation of inflammation and oligodendrogenesis. J. Am. Heart Assoc. 9, e013583 (2020).
Wang, Z. et al. The spleen may be an important target of stem cell therapy for stroke. J. Neuroinflammation 16, 20 (2019).
Rust, R. et al. Brain repair mechanisms after cell therapy for stroke. Brain 147, 3286–3305 (2024).
Hoseinzadeh, A. et al. Fate and long-lasting therapeutic effects of mesenchymal stromal/stem-like cells: mechanistic insights. Stem Cell Res. Ther. 16, 33 (2025).
Slama, Y. et al. The dual role of mesenchymal stem cells in cancer pathophysiology: pro-tumorigenic effects versus therapeutic potential. Int. J. Mol. Sci. 24, 13511 (2023).
Boshuizen, M. C. S. & Steinberg, G. K. Stem cell-based immunomodulation after stroke. Stroke 49, 1563–1570 (2018).
Liu, N. et al. Expression of IL-10 and TNF-α in rats with cerebral infarction after transplantation with mesenchymal stem cells. Cell Mol. Immunol. 6, 207–213 (2009).
Li, W. et al. Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death Differ. 19, 1505–1513 (2012).
Duijvestein, M. et al. Pretreatment with interferon-γ enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cell 29, 1549–1558 (2011).
Di Vincenzo, M. & Orciani, M. Special issue “The role of mesenchymal stem cells on inflammatory and fibrotic diseases”. Int. J. Mol. Sci. 24, 8578 (2023).
Wu, J.-P., Kuo, J.-S., Liu, Y.-L. & Tzeng, S.-F. Tumor necrosis factor-α modulates the proliferation of neural progenitors in the subventricular/ventricular zone of adult rat brain. Neurosci. Lett. 292, 203–206 (2000).
Heindl, S. et al. Automated morphological analysis of microglia after stroke. Front. Cell Neurosci. 12, 106 (2018).
Benkő, S. & Dénes, Á Microglial inflammatory mechanisms in stroke: the jury is still out. Neuroscience 550, 43–52 (2024).
Hu, X. et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 43, 3063–3070 (2012).
Xin, Q., Zhu, W., He, C., Liu, T. & Wang, H. The effect of different sources of mesenchymal stem cells on microglia states. Front. Aging Neurosci. 15, 1237532 (2023).
Chen, Y. et al. MSC-derived exosomes promote recovery from traumatic brain injury via microglia/macrophages in rat. Aging 12, 18274–18296 (2020).
Bai, X. J., Hao, L., Guo, Y. E., Shi, X. B. & Wu, W. P. Bone marrow stromal cells reverse the microglia type from pro-inflammatory tumour necrosis factor a microglia to anti-inflammatory CD206 microglia of middle cerebral artery occlusion rats through triggering secretion of CX3CL1. Folia Neuropathol. 59, 20–31 (2021).
Liu, X. et al. Bone marrow mesenchymal stem cell-derived exosomes attenuate cerebral ischemia-reperfusion injury-induced neuroinflammation and pyroptosis by modulating microglia M1/M2 phenotypes. Exp. Neurol. 341, 113700 (2021).
Ishizaka, S. et al. Intra-arterial cell transplantation provides timing-dependent cell distribution and functional recovery after stroke. Stroke 44, 720–726 (2013).
Gao, T., Huang, F., Wang, W., Xie, Y. & Wang, B. Interleukin-10 genetically modified clinical-grade mesenchymal stromal cells markedly reinforced functional recovery after spinal cord injury via directing alternative activation of macrophages. Cell Mol. Biol. Lett. 27, 27 (2022).
Song, Y. et al. M2 microglia extracellular vesicle miR-124 regulates neural stem cell differentiation in ischemic stroke via AAK1/NOTCH. Stroke 54, 2629–2639 (2023).
Vasandan, A. B. et al. Human mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE2-dependent mechanism. Sci. Rep. 6, 38308 (2016).
Osman, A. M. et al. The secretome of microglia regulate neural stem cell function. Neuroscience 405, 92–102 (2019).
Matsui, T. K. & Mori, E. Microglia support neural stem cell maintenance and growth. Biochem. Biophys. Res. Commun. 503, 1880–1884 (2018).
Popova, G. et al. Human microglia states are conserved across experimental models and regulate neural stem cell responses in chimeric organoids. Cell Stem Cell 28, 2153–2166.e2156 (2021).
Yu, B., Sondag, G. R., Malcuit, C., Kim, M. H. & Safadi, F. F. Macrophage-associated osteoactivin/GPNMB mediates mesenchymal stem cell survival, proliferation, and migration via a CD44-dependent mechanism. J. Cell Biochem. 117, 1511–1521 (2016).
Xia, Y. et al. Exosomes derived from M0, M1 and M2 macrophages exert distinct influences on the proliferation and differentiation of mesenchymal stem cells. PeerJ 8, e8970 (2020).
Nath, S. et al. Interaction between subventricular zone microglia and neural stem cells impacts the neurogenic response in a mouse model of cortical ischemic stroke. Nat. Commun. 15, 9095 (2024).
Jiang, X., Yi, S., Liu, Q. & Zhang, J. The secretome of microglia induced by IL-4 of IFN-γ differently regulate proliferation, differentiation and survival of adult neural stem/progenitor cell by targeting the PI3K–Akt pathway. Cytotechnology 74, 407–420 (2022).
Miró-Mur, F. et al. Antigen-dependent T cell response to neural peptides after human ischemic stroke. Front. Cell Neurosci. 14, 206 (2020).
Selvaraj, U. M. & Stowe, A. M. Long-term T cell responses in the brain after an ischemic stroke. Discov. Med. 24, 323–333 (2017).
Wang, Y.-R., Cui, W.-Q., Wu, H.-Y., Xu, X.-D. & Xu, X.-Q. The role of T cells in acute ischemic stroke. Brain Res. Bull. 196, 20–33 (2023).
Yilmaz, G., Arumugam, T. V., Stokes, K. Y. & Granger, D. N. Role of T lymphocytes and interferon-γ in ischemic stroke. Circulation 113, 2105–2112 (2006).
Shichita, T. et al. Pivotal role of cerebral interleukin-17-producing γδT cells in the delayed phase of ischemic brain injury. Nat. Med. 15, 946–950 (2009).
Di Nicola, M. et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99, 3838–3843 (2002).
Bartholomew, A. et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 30, 42–48 (2002).
Wang, D. et al. The regulation of the Treg/Th17 balance by mesenchymal stem cells in human systemic lupus erythematosus. Cell Mol. Immunol. 14, 423–431 (2017).
Bai, L. et al. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia 57, 1192–1203 (2009).
Yu, Y. Q. & Wang, H. Imbalance of Th1 and Th2 cytokines and stem cell therapy in pathological pain. CNS Neurol. Disord. Drug Targets 23, 88–101 (2024).
Xu, G., Zhang, Y., Zhang, L., Ren, G. & Shi, Y. The role of IL-6 in inhibition of lymphocyte apoptosis by mesenchymal stem cells. Biochem. Biophys. Res. Commun. 361, 745–750 (2007).
Ren, G. et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2, 141–150 (2008).
Fan, L. et al. Interaction between mesenchymal stem cells and B-Cells. Int. J. Mol. Sci. 17, 650 (2016).
Chiesa, S. et al. Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proc. Natl Acad. Sci. USA 108, 17384–17389 (2011).
Liu, J., Liu, Q. & Chen, X. The immunomodulatory effects of mesenchymal stem cells on regulatory B cells. Front. Immunol. 11, 1843 (2020).
Dulken, B. W. et al. Single-cell analysis reveals T cell infiltration in old neurogenic niches. Nature 571, 205–210 (2019).
Ziv, Y. et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat. Neurosci. 9, 268–275 (2006).
Benakis, C. et al. T cells modulate the microglial response to brain ischemia. eLife 11, e82031 (2022).
Schroeter, M. & Jander, S. T-cell cytokines in injury-induced neural damage and repair. Neuromol. Med. 7, 183–195 (2005).
Butovsky, O. et al. Microglia activated by IL-4 or IFN-γ differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol. Cell Neurosci. 31, 149–160 (2006).
Caplan, H. W. et al. Human cord blood-derived regulatory T-cell therapy modulates the central and peripheral immune response after traumatic brain injury. Stem Cell Transl. Med. 9, 903–916 (2020).
Sakaguchi, S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22, 531–562 (2004).
Curotto de Lafaille, M. A. & Lafaille, J. J. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity 30, 626–635 (2009).
Saino, O. et al. Immunodeficiency reduces neural stem/progenitor cell apoptosis and enhances neurogenesis in the cerebral cortex after stroke. J. Neurosci. Res. 88, 2385–2397 (2010).
Wang, J. et al. Activated regulatory T cell regulates neural stem cell proliferation in the subventricular zone of normal and ischemic mouse brain through interleukin 10. Front. Cell Neurosci. 9, 361 (2015).
Perez-Asensio, F. J., Perpiñá, U., Planas, A. M. & Pozas, E. Interleukin-10 regulates progenitor differentiation and modulates neurogenesis in adult brain. J. Cell Sci. 126, 4208–4219 (2013).
Brea, D. et al. Regulatory T cells modulate inflammation and reduce infarct volume in experimental brain ischaemia. J. Cell Mol. Med. 18, 1571–1579 (2014).
Na, S. Y., Mracsko, E., Liesz, A., Hünig, T. & Veltkamp, R. Amplification of regulatory T cells using a CD28 superagonist reduces brain damage after ischemic stroke in mice. Stroke 46, 212–220 (2015).
Li, P. et al. Adoptive regulatory T-cell therapy protects against cerebral ischemia. Ann. Neurol. 74, 458–471 (2013).
Liesz, A. et al. Boosting regulatory T cells limits neuroinflammation in permanent cortical stroke. J. Neurosci. 33, 17350–17362 (2013).
Kleinschnitz, C. et al. Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood 121, 679–691 (2013).
Ren, X., Akiyoshi, K., Vandenbark, A. A., Hurn, P. D. & Offner, H. CD4+FoxP3+ regulatory T-cells in cerebral ischemic stroke. Metab. Brain Dis. 26, 87–90 (2011).
Xu, X., Li, M. & Jiang, Y. The paradox role of regulatory T cells in ischemic stroke. ScientificWorldJournal 2013, 174373 (2013).
Caplan, H. W. et al. Human-derived Treg and MSC combination therapy may augment immunosuppressive potency in vitro, but did not improve blood brain barrier integrity in an experimental rat traumatic brain injury model. PLoS ONE 16, e0251601 (2021).
Caplan, H. W. et al. Combination therapy with Treg and mesenchymal stromal cells enhances potency and attenuation of inflammation after traumatic brain injury compared to monotherapy. Stem Cell 39, 358–370 (2021).
Lim, J. Y. et al. Enhanced immunoregulation of mesenchymal stem cells by IL-10-producing type 1 regulatory T cells in collagen-induced arthritis. Sci. Rep. 6, 26851 (2016).
Engela, A. U., Baan, C. C., Peeters, A. M., Weimar, W. & Hoogduijn, M. J. Interaction between adipose tissue-derived mesenchymal stem cells and regulatory T-cells. Cell Transpl. 22, 41–54 (2013).
Camacho, V. et al. Bone marrow Tregs mediate stromal cell function and support hematopoiesis via IL-10. JCI Insight 5, e135681 (2020).
Laffer, B. et al. Loss of IL-10 promotes differentiation of microglia to a M1 phenotype. Front. Cell Neurosci. 13, 430 (2019).
Lopes, R. L., Borges, T. J., Zanin, R. F. & Bonorino, C. IL-10 is required for polarization of macrophages to M2-like phenotype by mycobacterial DnaK (heat shock protein 70). Cytokine 85, 123–129 (2016).
Selmani, Z. et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cell 26, 212–222 (2008).
Greilach, S. A. et al. Presentation of human neural stem cell antigens drives regulatory T cell induction. J. Immunol. 210, 1677–1686 (2023).
Izzy, S. et al. Nasal anti-CD3 mAb ameliorates TBI, enhances microglia phagocytosis, and reduces neuroinflammation via IL-10-dependent Treg–microglia crosstalk. Nat. Neurosci. 28, 499–516 (2025).
Le Blanc, K. & Mougiakakos, D. Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 12, 383–396 (2012).
Coombes, J. L. et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J. Exp. Med. 204, 1757–1764 (2007).
Frenkel, D. et al. Nasal vaccination with myelin oligodendrocyte glycoprotein reduces stroke size by inducing IL-10-producing CD4+ T cells. J. Immunol. 171, 6549–6555 (2003).
Boroughs, A. C. et al. Chimeric antigen receptor costimulation domains modulate human regulatory T cell function. JCI Insight 5, e126194 (2019).
Baeten, P. et al. Rapamycin rescues loss of function in blood-brain barrier-interacting Tregs. JCI Insight 9, e167457 (2024).
Chandran, S. et al. Interleukin-6 blockade with tocilizumab increases Tregs and reduces T effector cytokines in renal graft inflammation: a randomized controlled trial. Am. J. Transplant. 21, 2543–2554 (2021).
Jung, M. K., Lee, J. S., Kwak, J. E. & Shin, E. C. Tumor necrosis factor and regulatory T Cells. Yonsei Med. J. 60, 126–131 (2019).
Shevach, E. M. Garp as a therapeutic target for modulation of T regulatory cell function. Expert Opin. Ther. Targets 21, 191–200 (2017).
Akkaya, B. et al. Regulatory T cells mediate specific suppression by depleting peptide–MHC class II from dendritic cells. Nat. Immunol. 20, 218–231 (2019).
Zhang, H. et al. In vivo expansion of regulatory T cells with IL-2/IL-2 antibody complex protects against transient ischemic stroke. J. Neurosci. 38, 10168–10179 (2018).
Huang, J. et al. Postischemic cerebrovascular E-selectin expression mediates tissue injury in murine stroke. Stroke 31, 3047–3053 (2000).
Faustman, D. L. & Davis, M. Stem cells in the spleen: therapeutic potential for Sjogren’s syndrome, type I diabetes, and other disorders. Int. J. Biochem. Cell Biol. 42, 1576–1579 (2010).
Seifert, H. A. et al. A transient decrease in spleen size following stroke corresponds to splenocyte release into systemic circulation. J. Neuroimmune Pharmacol. 7, 1017–1024 (2012).
Seifert, H. A. et al. The spleen contributes to stroke induced neurodegeneration through interferon gamma signaling. Metab. Brain Dis. 27, 131–141 (2012).
Ran, Y. et al. Splenectomy fails to provide long-term protection against ischemic stroke. Aging Dis. 9, 467–479 (2018).
Zheng, D., Bhuvan, T., Payne, N. L. & Heng, T. S. P. Secondary lymphoid organs in mesenchymal stromal cell therapy: more than just a filter. Front. Immunol. 13, 892443 (2022).
Acosta, S. A., Tajiri, N., Hoover, J., Kaneko, Y. & Borlongan, C. V. Intravenous bone marrow stem cell grafts preferentially migrate to spleen and abrogate chronic inflammation in stroke. Stroke 46, 2616–2627 (2015).
Keimpema, E. et al. Early transient presence of implanted bone marrow stem cells reduces lesion size after cerebral ischaemia in adult rats. Neuropathol. Appl. Neurobiol. 35, 89–102 (2009).
Yang, B. et al. Multipotent adult progenitor cells enhance recovery after stroke by modulating the immune response from the spleen. Stem Cell 35, 1290–1302 (2017).
Snyder, E. Y., Yoon, C., Flax, J. D. & Macklis, J. D. Multipotent neural precursors can differentiate toward replacement of neurons undergoing targeted apoptotic degeneration in adult mouse neocortex. Proc. Natl Acad. Sci. USA 94, 11663–11668 (1997).
Falkner, S. et al. Transplanted embryonic neurons integrate into adult neocortical circuits. Nature 539, 248–253 (2016).
Sakai, S. & Shichita, T. Inflammation and neural repair after ischemic brain injury. Neurochem. Int. 130, 104316 (2019).
Dai, J. et al. Migration of neural stem cells to ischemic brain regions in ischemic stroke in rats. Neurosci. Lett. 552, 124–128 (2013).
Palma-Tortosa, S. et al. Activity in grafted human iPS cell-derived cortical neurons integrated in stroke-injured rat brain regulates motor behavior. Proc. Natl Acad. Sci. USA 117, 9094–9100 (2020).
Green, C. et al. Sensorimotor functional and structural networks after intracerebral stem cell grafts in the ischemic mouse brain. J. Neurosci. 38, 1648–1661 (2018).
Tennstaedt, A. et al. Human neural stem cell intracerebral grafts show spontaneous early neuronal differentiation after several weeks. Biomaterials 44, 143–154 (2015).
Oki, K. et al. Human-induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem Cell 30, 1120–1133 (2012).
Tornero, D. et al. Human induced pluripotent stem cell-derived cortical neurons integrate in stroke-injured cortex and improve functional recovery. Brain 136, 3561–3577 (2013).
Wang, P. et al. Transplantation of human neural stem cells repairs neural circuits and restores neurological function in the stroke-injured brain. Neural Regen. Res. 21, 1162–1171 (2026).
Fesharaki, M. et al. Differentiation of human scalp adipose-derived mesenchymal stem cells into mature neural cells on electrospun nanofibrous scaffolds for nerve tissue engineering applications. Cell J. 20, 168–176 (2018).
Ma, Y. H. et al. Perineurium-like sheath derived from long-term surviving mesenchymal stem cells confers nerve protection to the injured spinal cord. Biomaterials 160, 37–55 (2018).
Zeng, X. et al. Integration of donor mesenchymal stem cell-derived neuron-like cells into host neural network after rat spinal cord transection. Biomaterials 53, 184–201 (2015).
Irisa, K. & Shichita, T. Neural repair mechanisms after ischemic stroke. Inflamm. Regen. 45, 7 (2025).
Stewart, A. N. et al. Nonresolving neuroinflammation regulates axon regeneration in chronic spinal cord injury. J. Neurosci. 45, e1017242024 (2025).
Freria, C. M. et al. Deletion of the Fractalkine receptor, CX3CR1, improves endogenous repair, axon sprouting, and synaptogenesis after spinal cord injury in mice. J. Neurosci. 37, 3568–3587 (2017).
Jakubs, K. et al. Inflammation regulates functional integration of neurons born in adult brain. J. Neurosci. 28, 12477–12488 (2008).
Solek, C. M. et al. Early inflammation dysregulates neuronal circuit formation in vivo via upregulation of IL-1β. J. Neurosci. 41, 6353–6366 (2021).
Wang, Y. et al. 3K3A-activated protein C stimulates postischemic neuronal repair by human neural stem cells in mice. Nat. Med. 22, 1050–1055 (2016).
Ma, H., Yu, B., Kong, L., Zhang, Y. & Shi, Y. Neural stem cells over-expressing brain-derived neurotrophic factor (BDNF) stimulate synaptic protein expression and promote functional recovery following transplantation in rat model of traumatic brain injury. Neurochem. Res. 37, 69–83 (2012).
Korshunova, I. et al. Genetic modification increases the survival and the neuroregenerative properties of transplanted neural stem cells. JCI Insight 5, e126268 (2020).
Gantner, C. W. et al. Viral delivery of GDNF promotes functional integration of human stem cell grafts in Parkinson’s disease. Cell Stem Cell 26, 511–526.e515 (2020).
He, J., Zhang, N., Zhu, Y., Jin, R. & Wu, F. MSC spheroids-loaded collagen hydrogels simultaneously promote neuronal differentiation and suppress inflammatory reaction through PI3K–Akt signaling pathway. Biomaterials 265, 120448 (2021).
Revah, O. et al. Maturation and circuit integration of transplanted human cortical organoids. Nature 610, 319–326 (2022).
Lo, R. Y. et al. Allogeneic human umbilical cord blood for acute ischemic stroke: phase I clinical trial. Tzu Chi Med. J. 37, 321–327 (2025).
Phan, T. G. et al. Phase I trial outcome of amnion cell therapy in patients with ischemic stroke (I-ACT). Front. Neurosci. 17, 1153231 (2023).
Chen, S. et al. Umbilical mesenchymal stem cells mitigate T-cell compartments shift and Th17/Treg imbalance in acute ischemic stroke via mitochondrial transfer. Stem Cell Res. Ther. 16, 134 (2025).
Kondziolka, D. et al. Transplantation of cultured human neuronal cells for patients with stroke. Neurology 55, 565–569 (2000).
Kondziolka, D. et al. Neurotransplantation for patients with subcortical motor stroke: a phase 2 randomized trial. J. Neurosurg. 103, 38–45 (2005).
Meltzer, C. C. et al. Serial [18F] fluorodeoxyglucose positron emission tomography after human neuronal implantation for stroke. Neurosurgery 49, 586–591 (2001).
Nelson, P. T. et al. Clonal human (hNT) neuron grafts for stroke therapy: neuropathology in a patient 27 months after implantation. Am. J. Pathol. 160, 1201–1206 (2002).
de Celis-Ruiz, E. et al. Allogeneic adipose tissue-derived mesenchymal stem cells in ischaemic stroke (AMASCIS-02): a phase IIb, multicentre, double-blind, placebo-controlled clinical trial protocol. BMJ Open 11, e051790 (2021).
US National library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03570450 (2025).
Wheeler, M. A. & Quintana, F. J. The neuroimmune connectome in health and disease. Nature 638, 333–342 (2025).
Wang, Z. et al. Imaging of microglia in post-stroke inflammation. Nucl. Med. Biol. 118-119, 108336 (2023).
Cao, S.-Y. et al. Cerebral organoids transplantation repairs infarcted cortex and restores impaired function after stroke. npj Regen. Med. 8, 27 (2023).
Boufidis, D., Garg, R., Angelopoulos, E., Cullen, D. K. & Vitale, F. Bio-inspired electronics: Soft, biohybrid, and “living” neural interfaces. Nat. Commun. 16, 1861 (2025).
Cheng, M. Y. et al. Optogenetic neuronal stimulation promotes functional recovery after stroke. Proc. Natl Acad. Sci. USA 111, 12913–12918 (2014).
Li, P. et al. Engineered extracellular vesicles for ischemic stroke: a systematic review and meta-analysis of preclinical studies. J. Nanobiotechnol. 21, 396 (2023).
Birch, L. A. Engineering extracellular vesicles for therapeutic delivery in ischaemic stroke. Nat. Rev. Cardiol. 22, 219–219 (2025).
Oliveira, J. M. et al. Hydrogel-based scaffolds to support intrathecal stem cell transplantation as a gateway to the spinal cord: clinical needs, biomaterials, and imaging technologies. npj Regen. Med. 3, 8 (2018).
Cotrina, M. L., Lou, N., Tome-Garcia, J., Goldman, J. & Nedergaard, M. Direct comparison of microglial dynamics and inflammatory profile in photothrombotic and arterial occlusion evoked stroke. Neuroscience 343, 483–494 (2017).
Hou, B. et al. Exogenous neural stem cells transplantation as a potential therapy for photothrombotic ischemia stroke in kunming mice model. Mol. Neurobiol. 54, 1254–1262 (2017).
Abeysinghe, H. C., Bokhari, L., Dusting, G. J. & Roulston, C. L. Brain remodelling following endothelin-1 induced stroke in conscious rats. PLoS ONE 9, e97007 (2014).
Modo, M. M., Jolkkonen, J., Zille, M. & Boltze, J. Future of animal modeling for poststroke tissue repair. Stroke 49, 1099–1106 (2018).
Burns, T. C. & Steinberg, G. K. Stem cells and stroke: opportunities, challenges and strategies. Expert Opin. Biol. Ther. 11, 447–461 (2011).
Janowski, M., Walczak, P. & Date, I. Intravenous route of cell delivery for treatment of neurological disorders: a meta-analysis of preclinical results. Stem Cell Dev. 19, 5–16 (2010).
Dharmasaroja, P. Bone marrow-derived mesenchymal stem cells for the treatment of ischemic stroke. J. Clin. Neurosci. 16, 12–20 (2009).
Cerneckis, J., Cai, H. & Shi, Y. Induced pluripotent stem cells (iPSCs): molecular mechanisms of induction and applications. Signal Transduct. Target. Ther. 9, 112 (2024).
Pereira, I. M., Marote, A., Salgado, A. J. & Silva, N. A. Filling the gap: neural stem cells as a promising therapy for spinal cord injury. Pharmaceuticals 12, 65 (2019).
Zhang, R. et al. NSC-derived exosomes enhance therapeutic effects of NSC transplantation on cerebral ischemia in mice. eLife 12, e84493 (2023).
Weber, R. Z. et al. Neural xenografts contribute to long-term recovery in stroke via molecular graft–host crosstalk. Nat. Commun. 16, 8224 (2025).
Zhang, W. et al. Poststroke intravenous transplantation of human mesenchymal stem cells improves brain repair dynamics and functional outcomes in aged mice. Stroke 54, 1088–1098 (2023).
Yang, Y. et al. Human umbilical cord derived mesenchymal stem cells overexpressing HO-1 attenuate neural injury and enhance functional recovery by inhibiting inflammation in stroke mice. CNS Neurosci. Ther. 30, e14412 (2024).
Horie, N. et al. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cell 29, 274–285 (2011).
Hassani, Z. et al. Human neural progenitor cell engraftment increases neurogenesis and microglial recruitment in the brain of rats with stroke. PLoS ONE 7, e50444 (2012).
Chang, D.-J. et al. Contralaterally transplanted human embryonic stem cell-derived neural precursor cells (ENStem-A) migrate and improve brain functions in stroke-damaged rats. Exp. Mol. Med. 45, e53 (2013).
Chang, D.-J. et al. Therapeutic potential of human induced pluripotent stem cells in experimental stroke. Cell Transplant. 22, 1427–1440 (2013).
Watanabe, T. et al. A human neural stem cell line provides neuroprotection and improves neurological performance by early intervention of neuroinflammatory system. Brain Res. 1631, 194–203 (2016).
Vendrame, M. et al. Anti-inflammatory effects of human cord blood cells in a rat model of stroke. Stem Cell Dev. 14, 595–604 (2005).
Li, Y. et al. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia 49, 407–417 (2005).
Wei, L., Fraser, J. L., Lu, Z. Y., Hu, X. & Yu, S. P. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol. Dis. 46, 635–645 (2012).
McGuckin, C. P. et al. Ischemic brain injury: a consortium analysis of key factors involved in mesenchymal stem cell-mediated inflammatory reduction. Arch. Biochem. Biophys. 534, 88–97 (2013).
Xin, H. et al. Multipotent mesenchymal stromal cells decrease transforming growth factor β1 expression in microglia/macrophages and down-regulate plasminogen activator inhibitor 1 expression in astrocytes after stroke. Neurosci. Lett. 542, 81–86 (2013).
Tsai, M. J. et al. Recovery of neurological function of ischemic stroke by application of conditioned medium of bone marrow mesenchymal stem cells derived from normal and cerebral ischemia rats. J. Biomed. Sci. 21, 5 (2014).
Lin, W. et al. Human umbilical cord mesenchymal stem cells preserve adult newborn neurons and reduce neurological injury after cerebral ischemia by reducing the number of hypertrophic microglia/macrophages. Cell Transpl. 26, 1798–1810 (2017).
Nakajima, M. et al. Mesenchymal stem cells overexpressing interleukin-10 promote neuroprotection in experimental acute ischemic stroke. Mol. Ther. Methods Clin. Dev. 6, 102–111 (2017).
Jiang, M. et al. Exosomes from MiR-30d-5p-ADSCs reverse acute ischemic stroke-induced, autophagy-mediated brain injury by promoting M2 microglial/macrophage polarization. Cell Physiol. Biochem. 47, 864–878 (2018).
Kong, T. et al. Immunomodulatory effect of CD200-positive human placenta-derived stem cells in the early phase of stroke. Exp. Mol. Med. 50, e425 (2018).
Dabrowska, S. et al. Human bone marrow mesenchymal stem cell-derived extracellular vesicles attenuate neuroinflammation evoked by focal brain injury in rats. J. Neuroinflammation 16, 216 (2019).
Geng, W. et al. Exosomes from miRNA-126-modified ADSCs promotes functional recovery after stroke in rats by improving neurogenesis and suppressing microglia activation. Am. J. Transl. Res. 11, 780–792 (2019).
Gómez-de Frutos, M. C. et al. Intravenous delivery of adipose tissue-derived mesenchymal stem cells improves brain repair in hyperglycemic stroke rats. Stem Cell Res. Ther. 10, 212 (2019).
Li, Z. et al. Bone marrow-mesenchymal stem cells modulate microglial activation in the peri-infarct area in rats during the acute phase of stroke. Brain Res. Bull. 153, 324–333 (2019).
Tatebayashi, K. et al. Adipose-derived stem cell therapy inhibits the deterioration of cerebral infarction by altering macrophage kinetics. Brain Res. 1712, 139–150 (2019).
Feng, Y. W. et al. hUCMSCs mitigate LPS-induced trained immunity in ischemic stroke. Front. Immunol. 11, 1746 (2020).
Yang, F. et al. Bone marrow mesenchymal stem cells induce M2 microglia polarization through PDGF–AA/MANF signaling. World J. Stem Cell 12, 633–658 (2020).
Zhao, Y., Gan, Y., Xu, G., Hua, K. & Liu, D. Exosomes from MSCs overexpressing microRNA-223-3p attenuate cerebral ischemia through inhibiting microglial M1 polarization mediated inflammation. Life Sci. 260, 118403 (2020).
Hu, X. et al. Extracellular vesicles from adipose-derived stem cells promote microglia M2 polarization and neurological recovery in a mouse model of transient middle cerebral artery occlusion. Stem Cell Res. Ther. 13, 21 (2022).
Li, Y. et al. Three-dimensional cultured mesenchymal stem cells enhance repair of ischemic stroke through inhibition of microglia. Stem Cell Res. Ther. 12, 358 (2021).
Pathipati, P. et al. Mesenchymal stem cell (MSC)-derived extracellular vesicles protect from neonatal stroke by interacting with microglial cells. Neurotherapeutics 18, 1939–1952 (2021).
Zhang, Z. et al. Human umbilical cord mesenchymal stem cell-derived exosomal miR-146a-5p reduces microglial-mediated neuroinflammation via suppression of the IRAK1/TRAF6 signaling pathway after ischemic stroke. Aging 13, 3060–3079 (2021).
Acknowledgements
Figures were created with BioRender (https://www.biorender.com).
Author information
Authors and Affiliations
Contributions
N.M., A.M., P.K., T.Y., R.T. and S.I. researched data for the article. N.M., A.M., H.W., S.S., D.Y. and S.F. provided substantial contributions to the discussion of its content. The authors all contributed to writing the article and reviewing and editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neuroscience thanks Zaal Kokaia, who co-reviewed with Sara Palma-Tortosa, Jean-Pyo Lee and Fu-Dong Shi for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McMillan, N., McMillan, A., Kiliaan, P. et al. Stem cell-mediated recovery in stroke: partnering with the immune system. Nat. Rev. Neurosci. 27, 23–43 (2026). https://doi.org/10.1038/s41583-025-00985-4
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41583-025-00985-4