Abstract
Damage to articular cartilage, tendons, ligaments and entheses as a result of trauma, degeneration or inflammation in rheumatic diseases is prevalent. Regenerative medicine offers promising strategies for repairing damaged tissues, with the aim of restoring both their structure and function. While these strategies have traditionally relied on tissue engineering approaches using exogenous cells, interventions based on the activation of endogenous repair mechanisms are an attractive alternative. Key to advancing such approaches is a comprehensive understanding of the diversity of the stem and progenitor cells that reside in the adult synovial joint and how they function to repair damaged tissues. Advances in developmental biology have provided a lens through which to understand the origins, identities and functions of these cells, and insights into the roles of stem and progenitor cells in joint tissue repair, as well as their complex relationship with fibroblasts, have emerged. Integration of knowledge obtained through studies using advanced single-cell technologies will be crucial to establishing unified models of cell populations, lineage hierarchies and their molecular regulation. Ultimately, a more complete understanding of how cells repair tissues in adult life will guide the development of innovative pro-regenerative drugs, which are poised to enter clinical practice in musculoskeletal medicine.
Key points
-
Joint tissues are susceptible to damage that often does not adequately heal without intervention and can predispose to osteoarthritis.
-
Understanding the regenerative biology of the synovial joint will guide the development of therapeutic strategies to activate endogenous repair mechanisms and improve outcomes.
-
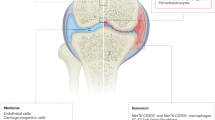
In adult joint tissues, stem and progenitor cell niches are present in the synovial lining and sublining, the paratenon and tendon sheath, the superficial zone of cartilage and the subchondral bone marrow.
-
The identity and functions of the different stem and progenitor cell populations in adult joint tissues can be understood in the context of their diverse developmental origins.
-
The synovium could be a reservoir of joint-repairing cells, and cells from bone marrow can contribute to the repair of osteochondral defects.
-
Single-cell technologies offer the opportunity to establish integrated models of cell populations and lineage hierarchies and their molecular regulation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hjelle, K., Solheim, E., Strand, T., Muri, R. & Brittberg, M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy 18, 730–734 (2002).
Widuchowski, W., Widuchowski, J. & Trzaska, T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee 14, 177–182 (2007).
Hunter, D. J. & Bierma-Zeinstra, S. Osteoarthritis. Lancet 393, 1745–1759 (2019).
Weng, Q. et al. Global burden of early-onset osteoarthritis, 1990–2019: results from the Global Burden of Disease Study 2019. Ann. Rheum. Dis. 83, 915–925 (2024).
GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1545–1602 (2016).
De Bari, C. & Roelofs, A. J. Stem cell-based therapeutic strategies for cartilage defects and osteoarthritis. Curr. Opin. Pharmacol. 40, 74–80 (2018).
Dell’accio, F. & Vincent, T. L. Joint surface defects: clinical course and cellular response in spontaneous and experimental lesions. Eur. Cell Mater. 20, 210–217 (2010).
Jansen, M. P. & Mastbergen, S. C. Joint distraction for osteoarthritis: clinical evidence and molecular mechanisms. Nat. Rev. Rheumatol. 18, 35–46 (2022).
Eltawil, N. M., De Bari, C., Achan, P., Pitzalis, C. & Dell’accio, F. A novel in vivo murine model of cartilage regeneration. Age and strain-dependent outcome after joint surface injury. Osteoarthritis Cartilage 17, 695–704 (2009).
Rai, M. F. et al. Heritability of articular cartilage regeneration and its association with ear wound healing in mice. Arthritis Rheum. 64, 2300–2310 (2012).
Thorup, A.-S., Dell’Accio, F. & Eldridge, S. E. Lessons from joint development for cartilage repair in the clinic. Dev. Dyn. 250, 360–376 (2021).
Pineault, K. M. & Wellik, D. M. Hox genes and limb musculoskeletal development. Curr. Osteoporos. Rep. 12, 420–427 (2014).
Pineault, K. M., Song, J. Y., Kozloff, K. M., Lucas, D. & Wellik, D. M. Hox11 expressing regional skeletal stem cells are progenitors for osteoblasts, chondrocytes and adipocytes throughout life. Nat. Commun. 10, 3168 (2019).
Rux, D. R. et al. Regionally restricted Hox function in adult bone marrow multipotent mesenchymal stem/stromal cells. Dev. Cell 39, 653–666 (2016).
Gronthos, S., Graves, S. E., Ohta, S. & Simmons, P. J. The STRO-1+ fraction of adult human bone marrow contains the osteogenic precursors. Blood 84, 4164–4173 (1994).
Jones, E. A. et al. Optimization of a flow cytometry-based protocol for detection and phenotypic characterization of multipotent mesenchymal stromal cells from human bone marrow. Cytometry B Clin. Cytom. 70, 391–399 (2006).
Sacchetti, B. et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131, 324–336 (2007).
Pinho, S. et al. PDGFRα and CD51 mark human Nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J. Exp. Med. 210, 1351–1367 (2013).
Chan, C. K. F. et al. Identification of the human skeletal stem cell. Cell 175, 43–56.e21 (2018).
Morikawa, S. et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J. Exp. Med. 206, 2483–2496 (2009).
Méndez-Ferrer, S. et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834 (2010).
Isern, J. et al. The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function. Elife 3, e03696 (2014).
Ding, L., Saunders, T. L., Enikolopov, G. & Morrison, S. J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462 (2012).
Zhou, B. O., Yue, R., Murphy, M. M., Peyer, J. G. & Morrison, S. J. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15, 154–168 (2014).
Worthley, D. L. et al. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell 160, 269–284 (2015).
Kfoury, Y. & Scadden, D. T. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell 16, 239–253 (2015).
Chan, C. K. F. et al. Identification and specification of the mouse skeletal stem cell. Cell 160, 285–298 (2015).
Ambrosi, T. H. et al. Distinct skeletal stem cell types orchestrate long bone skeletogenesis. Elife 10, e66063 (2021).
Shu, H. S. et al. Tracing the skeletal progenitor transition during postnatal bone formation. Cell Stem Cell 28, 2122–2136.e3 (2021).
Matsushita, Y. et al. Bone marrow endosteal stem cells dictate active osteogenesis and aggressive tumorigenesis. Nat. Commun. 14, 2383 (2023).
Murphy, M. P. et al. Articular cartilage regeneration by activated skeletal stem cells. Nat. Med. 26, 1583–1592 (2020).
Ambrosi, T. H. et al. Aged skeletal stem cells generate an inflammatory degenerative niche. Nature 597, 256–262 (2021).
Mizoguchi, T. et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev. Cell 29, 340–349 (2014).
Massengale, M. et al. Adult Prg4+ progenitors repair long-term articular cartilage wounds in vivo. JCI Insight 8, e167858 (2023).
Kurth, T. B. et al. Functional mesenchymal stem cell niches in adult mouse knee joint synovium in vivo. Arthritis Rheum. 63, 1289–1300 (2011).
Rountree, R. B. et al. BMP receptor signaling is required for postnatal maintenance of articular cartilage. PLoS Biol. 2, e355 (2004).
Koyama, E. et al. A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev. Biol. 316, 62–73 (2008).
Roelofs, A. J. et al. Joint morphogenetic cells in the adult mammalian synovium. Nat. Commun. 8, 15040 (2017).
Collins, F. L. et al. Taxonomy of fibroblasts and progenitors in the synovial joint at single-cell resolution. Ann. Rheum. Dis. 82, 428–437 (2023).
Decker, R. S., Koyama, E. & Pacifici, M. Genesis and morphogenesis of limb synovial joints and articular cartilage. Matrix Biol. 39, 5–10 (2014).
Shwartz, Y., Viukov, S., Krief, S. & Zelzer, E. Joint development involves a continuous influx of gdf5-positive cells. Cell Rep. 15, 2577–2587 (2016).
Chen, H. et al. Heads, shoulders, elbows, knees, and toes: modular Gdf5 enhancers control different joints in the vertebrate skeleton. PLoS Genet. 12, e1006454 (2016).
Pregizer, S. K. et al. Impact of broad regulatory regions on Gdf5 expression and function in knee development and susceptibility to osteoarthritis. Ann. Rheum. Dis. 77, 450 (2018).
Kania, K. et al. Regulation of Gdf5 expression in joint remodelling, repair and osteoarthritis. Sci. Rep. 10, 157 (2020).
Dowthwaite, G. P. et al. The surface of articular cartilage contains a progenitor cell population. J. Cell Sci. 117, 889–897 (2004).
Kozhemyakina, E. et al. Identification of a Prg4-expressing articular cartilage progenitor cell population in mice. Arthritis Rheumatol. 67, 1261–1273 (2015).
Li, L. et al. Superficial cells are self-renewing chondrocyte progenitors, which form the articular cartilage in juvenile mice. FASEB J. 31, 1067–1084 (2017).
Decker, R. S. et al. Cell origin, volume and arrangement are drivers of articular cartilage formation, morphogenesis and response to injury in mouse limbs. Dev. Biol. 426, 56–68 (2017).
Roelofs, A. J. et al. Identification of the skeletal progenitor cells forming osteophytes in osteoarthritis. Ann. Rheum. Dis. 79, 1625–1634 (2020).
Debnath, S. et al. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature 562, 133–139 (2018).
Bechtold, T. E. et al. Osteophyte formation and matrix mineralization in a TMJ osteoarthritis mouse model are associated with ectopic hedgehog signaling. Matrix Biol. 52–54, 339–354 (2016).
Maes, C. et al. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev. Cell 19, 329–344 (2010).
Xie, M. & Chagin, A. S. The epiphyseal secondary ossification center: evolution, development and function. Bone 142, 115701 (2021).
Tong, W. et al. Periarticular mesenchymal progenitors initiate and contribute to secondary ossification center formation during mouse long bone development. Stem Cell 37, 677–689 (2019).
Schett, G. et al. Enthesitis: from pathophysiology to treatment. Nat. Rev. Rheumatol. 13, 731–741 (2017).
Blitz, E., Sharir, A., Akiyama, H. & Zelzer, E. Tendon-bone attachment unit is formed modularly by a distinct pool of Scx- and Sox9-positive progenitors. Development 140, 2680–2690 (2013).
Sugimoto, Y. et al. Scx+/Sox9+ progenitors contribute to the establishment of the junction between cartilage and tendon/ligament. Development 140, 2280–2288 (2013).
Eyal, S. et al. On the development of the patella. Development 142, 1831–1839 (2015).
Eyal, S. et al. Bone morphology is regulated modularly by global and regional genetic programs. Development 146, dev167882 (2019).
Eyal, S., Rubin, S., Krief, S., Levin, L. & Zelzer, E. Common cellular origin and diverging developmental programs for different sesamoid bones. Development 146, dev167452 (2019).
Blitz, E. et al. Bone ridge patterning during musculoskeletal assembly is mediated through SCX regulation of Bmp4 at the tendon-skeleton junction. Dev. Cell 17, 861–873 (2009).
Roberts, R. R. et al. FGF signaling patterns cell fate at the interface between tendon and bone. Development 146, dev170241 (2019).
Ono, N. & Kronenberg, H. M. Mesenchymal progenitor cells for the osteogenic lineage. Curr. Mol. Biol. Rep. 1, 95–100 (2015).
Arostegui, M., Scott, R. W., Böse, K. & Underhill, T. M. Cellular taxonomy of Hic1+ mesenchymal progenitor derivatives in the limb: from embryo to adult. Nat. Commun. 13, 4989 (2022).
Arostegui, M., Scott, R. W. & Underhill, T. M. Hic1 identifies a specialized mesenchymal progenitor population in the embryonic limb responsible for bone superstructure formation. Cell Rep. 42, 112325 (2023).
Dyment, N. A. et al. Lineage tracing of resident tendon progenitor cells during growth and natural healing. PLoS ONE 9, e96113 (2014).
Dyment, N. A. et al. Gdf5 progenitors give rise to fibrocartilage cells that mineralize via hedgehog signaling to form the zonal enthesis. Dev. Biol. 405, 96–107 (2015).
Schwartz, A. G., Long, F. & Thomopoulos, S. Enthesis fibrocartilage cells originate from a population of Hedgehog-responsive cells modulated by the loading environment. Development 142, 196–206 (2015).
Zhang, T. et al. Single-cell RNA sequencing reveals cellular and molecular heterogeneity in fibrocartilaginous enthesis formation. Elife 12, e85873 (2023).
Liu, C.-F., Breidenbach, A., Aschbacher-Smith, L., Butler, D. & Wylie, C. A role for hedgehog signaling in the differentiation of the insertion site of the patellar tendon in the mouse. PLoS ONE 8, e65411 (2013).
Felsenthal, N. et al. Development of migrating tendon-bone attachments involves replacement of progenitor populations. Development 145, dev165381 (2018).
Fang, F., Schwartz, A. G., Moore, E. R., Sup, M. E. & Thomopoulos, S. Primary cilia as the nexus of biophysical and hedgehog signaling at the tendon enthesis. Sci. Adv. 6, eabc1799 (2020).
Schwartz, A. G., Galatz, L. M. & Thomopoulos, S. Enthesis regeneration: a role for Gli1+ progenitor cells. Development 144, 1159–1164 (2017).
Fang, F., Xiao, Y., Zelzer, E., Leong, K. W. & Thomopoulos, S. A mineralizing pool of Gli1-expressing progenitors builds the tendon enthesis and demonstrates therapeutic potential. Cell Stem Cell 29, 1669–1684.e6 (2022).
Xiao, H. et al. Mechanical stimulation promotes enthesis injury repair by mobilizing Prrx1+ cells via ciliary TGF-β signaling. Elife 11, e73614 (2022).
Bi, Y. et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 13, 1219–1227 (2007).
Harvey, T., Flamenco, S. & Fan, C.-M. A Tppp3+Pdgfra+ tendon stem cell population contributes to regeneration and reveals a shared role for PDGF signalling in regeneration and fibrosis. Nat. Cell Biol. 21, 1490–1503 (2019).
Tachibana, N. et al. RSPO2 defines a distinct undifferentiated progenitor in the tendon/ligament and suppresses ectopic ossification. Sci. Adv. 8, eabn2138 (2022).
Wang, Y. et al. Osteocalcin expressing cells from tendon sheaths in mice contribute to tendon repair by activating Hedgehog signaling. Elife 6, e30474 (2017).
Yea, J.-H. et al. Tppp3+ synovial/tendon sheath progenitor cells contribute to heterotopic bone after trauma. Bone Res. 11, 39 (2023).
Dyment, N. A. et al. The paratenon contributes to scleraxis-expressing cells during patellar tendon healing. PLoS ONE 8, e59944 (2013).
Sakabe, T. et al. Transcription factor scleraxis vitally contributes to progenitor lineage direction in wound healing of adult tendon in mice. J. Biol. Chem. 293, 5766–5780 (2018).
Liu, W. et al. The atypical homeodomain transcription factor Mohawk controls tendon morphogenesis. Mol. Cell Biol. 30, 4797–4807 (2010).
Buechler, M. B. et al. Cross-tissue organization of the fibroblast lineage. Nature 593, 575–579 (2021).
Knights, A. J. et al. Synovial fibroblasts assume distinct functional identities and secrete R-spondin 2 in osteoarthritis. Ann. Rheum. Dis. 82, 272–282 (2023).
Li, J. et al. Synovium and infrapatellar fat pad share common mesenchymal progenitors and undergo coordinated changes in osteoarthritis. J. Bone Miner. Res. 39, 161–176 (2024).
Tang, S. et al. Single-cell atlas of human infrapatellar fat pad and synovium implicates APOE signaling in osteoarthritis pathology. Sci. Transl. Med. 16, eadf4590 (2024).
Clevers, H. & Watt, F. M. Defining adult stem cells by function, not by phenotype. Annu. Rev. Biochem. 87, 1015–1027 (2018).
Ng, J. Q. et al. Loss of Grem1-lineage chondrogenic progenitor cells causes osteoarthritis. Nat. Commun. 14, 6909 (2023).
Matsuzaki, T. et al. FoxO transcription factors modulate autophagy and proteoglycan 4 in cartilage homeostasis and osteoarthritis. Sci. Transl. Med. 10, eaan0746 (2018).
Wang, C., Shen, J., Ying, J., Xiao, D. & O’Keefe, R. J. FoxO1 is a crucial mediator of TGF-β/TAK1 signaling and protects against osteoarthritis by maintaining articular cartilage homeostasis. Proc. Natl Acad. Sci. USA 117, 30488–30497 (2020).
Lefebvre, V. Roles and regulation of SOX transcription factors in skeletogenesis. Curr. Top. Dev. Biol. 133, 171–193 (2019).
van Gastel, N. et al. Lipid availability determines fate of skeletal progenitor cells via SOX9. Nature 579, 111–117 (2020).
Croft, A. P. et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature 570, 246–251 (2019).
Vincent, T. L. Of mice and men: converging on a common molecular understanding of osteoarthritis. Lancet Rheumatol. 2, e633–e645 (2020).
Lohmander, L. S. et al. Intraarticular sprifermin (recombinant human fibroblast growth factor 18) in knee osteoarthritis: a randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 66, 1820–1831 (2014).
Hochberg, M. C. et al. Effect of intra-articular sprifermin vs placebo on femorotibial joint cartilage thickness in patients with osteoarthritis: the FORWARD randomized clinical trial. JAMA 322, 1360–1370 (2019).
Guehring, H. et al. The effects of sprifermin on symptoms and structure in a subgroup at risk of progression in the FORWARD knee osteoarthritis trial. Semin. Arthritis Rheum. 51, 450–456 (2021).
Eckstein, F. et al. Long-term structural and symptomatic effects of intra-articular sprifermin in patients with knee osteoarthritis: 5-year results from the FORWARD study. Ann. Rheum. Dis. 80, 1062–1069 (2021).
Deshmukh, V. et al. A small-molecule inhibitor of the Wnt pathway (SM04690) as a potential disease modifying agent for the treatment of osteoarthritis of the knee. Osteoarthritis Cartilage 26, 18–27 (2018).
Yazici, Y. et al. A novel Wnt pathway inhibitor, SM04690, for the treatment of moderate to severe osteoarthritis of the knee: results of a 24-week, randomized, controlled, phase 1 study. Osteoarthritis Cartilage 25, 1598–1606 (2017).
Yazici, Y. et al. Lorecivivint, a novel intraarticular CDC-like kinase 2 and dual-specificity tyrosine phosphorylation-regulated kinase 1a inhibitor and Wnt pathway modulator for the treatment of knee osteoarthritis: a phase II randomized trial. Arthritis Rheumatol. 72, 1694–1706 (2020).
Gerwin, N. et al. Angiopoietin-like 3-derivative LNA043 for cartilage regeneration in osteoarthritis: a randomized phase 1 trial. Nat. Med. 28, 2633–2645 (2022).
Eldridge, S. E. et al. Agrin induces long-term osteochondral regeneration by supporting repair morphogenesis. Sci. Transl. Med. 12, eaax9086 (2020).
Ruscitto, A. et al. Lgr5-expressing secretory cells form a Wnt inhibitory niche in cartilage critical for chondrocyte identity. Cell Stem Cell 30, 1179–1198.e7 (2023).
Zelinka, A., Roelofs, A. J., Kandel, R. A. & De Bari, C. Cellular therapy and tissue engineering for cartilage repair. Osteoarthritis Cartilage 30, 1547–1560 (2022).
Kunisaki, Y. et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 502, 637–643 (2013).
Arranz, L. et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature 512, 78–81 (2014).
Shen, B. et al. A mechanosensitive peri-arteriolar niche for osteogenesis and lymphopoiesis. Nature 591, 438–444 (2021).
Mizuhashi, K. et al. Resting zone of the growth plate houses a unique class of skeletal stem cells. Nature 563, 254–258 (2018).
Muruganandan, S. et al. A FoxA2+ long-term stem cell population is necessary for growth plate cartilage regeneration after injury. Nat. Commun. 13, 2515 (2022).
Brittberg, M. et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 331, 889–895 (1994).
Ogura, T., Mosier, B. A., Bryant, T. & Minas, T. A 20-year follow-up after first-generation autologous chondrocyte implantation. Am. J. Sports Med. 45, 2751–2761 (2017).
Steadman, J. R., Rodkey, W. G. & Rodrigo, J. J. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin. Orthop. Relat. Res. S362–S369 (2001).
Saris, D. B. F. et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am. J. Sports Med. 36, 235–246 (2008).
Saris, D. B. F. et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am. J. Sports Med. 37, 10S–19S (2009).
Vanlauwe, J. et al. Five-year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee: early treatment matters. Am. J. Sports Med. 39, 2566–2574 (2011).
Knutsen, G. et al. A randomized multicenter trial comparing autologous chondrocyte implantation with microfracture: long-term follow-up at 14 to 15 years. J. Bone Jt. Surg. Am. 98, 1332–1339 (2016).
Hoburg, A. et al. Sustained superiority in KOOS subscores after matrix-associated chondrocyte implantation using spheroids compared to microfracture. Knee Surg. Sports Traumatol. Arthrosc. 31, 2482–2493 (2023).
Barry, F. & Murphy, M. Mesenchymal stem cells in joint disease and repair. Nat. Rev. Rheumatol. 9, 584–594 (2013).
Zhang, Y., Yang, H., He, F. & Zhu, X. Intra-articular injection choice for osteoarthritis: making sense of cell source-an updated systematic review and dual network meta-analysis. Arthritis Res. Ther. 24, 260 (2022).
Mautner, K. et al. Cell-based versus corticosteroid injections for knee pain in osteoarthritis: a randomized phase 3 trial. Nat. Med. 29, 3120–3126 (2023).
Author information
Authors and Affiliations
Contributions
All authors researched data for and wrote the article. C.D.B. and A.J.R. contributed substantially to discussion of the content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
A.J.R. and C.D.B. have received research grant funding through their institution from Biosplice Therapeutics (formerly Samumed). C.D.B. declares that he has received consultancy fees from Celltrion Healthcare, Galapagos and UCB. J.J.M. and E.A.H. declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks Lucienne Vonk and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roelofs, A.J., McClure, J.J., Hay, E.A. et al. Stem and progenitor cells in the synovial joint as targets for regenerative therapy. Nat Rev Rheumatol 21, 211–220 (2025). https://doi.org/10.1038/s41584-025-01222-z
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41584-025-01222-z
This article is cited by
-
Dickkopf-3 enhances the chondroprotective effect of chondrogenic progenitor cells in a rat model of posttraumatic osteoarthritis
Journal of Orthopaedic Surgery and Research (2025)