Abstract
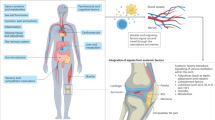
Osteoarthritis (OA) is a chronic joint disease that has long been considered a simple wear-and-tear condition. Over the past decade, research has revealed that various inflammatory features of OA, such as low-grade peripheral inflammation and synovitis, contribute substantially to the pathophysiology of the disease. Technological advances in the past 5 years have revealed a large diversity of innate and adaptive immune cells in the joints, particularly in the synovium and infrapatellar fat pad. Notably, the presence of synovial lymphoid structures, circulating autoantibodies and alterations in memory T cell and B cell populations have been documented in OA. These data indicate a potential contribution of self-reactivity to the disease pathogenesis, blurring the often narrow and inaccurate line between chronic inflammatory and autoimmune diseases. The diverse immune changes associated with OA pathogenesis can vary across disease phenotypes, and a better characterization of their underlying molecular endotypes will be key to stratifying patients, designing novel therapeutic approaches and ultimately ameliorating treatment allocation. Furthermore, examining both articular and systemic alterations, including changes in the gut–joint axis and microbial dysbiosis, could open up novel avenues for OA management.
Key points
-
Technological advances, such as single-cell RNA sequencing, have revealed an unexpected diversity of immune cells within joint tissues in osteoarthritis (OA), particularly in the synovium and infrapatellar fat pad.
-
At advanced stages of OA, aggregates that comprise B cells and T cells surrounded by plasma cells are observed in synovial tissues.
-
The presence of circulating autoantibodies and alterations in memory T cell and B cell populations that are reported in OA indicate a potential contribution of self-reactivity to disease pathogenesis.
-
Immune changes in OA contribute to both articular and systemic low-grade inflammation, the underlying mechanisms of which might vary depending on the OA phenotype, representing potential clinically relevant therapeutic targets.
-
The gut microbiota and associated immune responses have a role in OA pathophysiology, and potentially represent novel therapeutic targets.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Safiri, S. et al. Global, regional and national burden of osteoarthritis 1990–2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann. Rheum. Dis. 79, 819–828 (2020).
Price, A. J. et al. Knee replacement. Lancet 392, 1672–1682 (2018).
Ferguson, R. J. et al. Hip replacement. Lancet 392, 1662–1671 (2018).
Osteoarthritis Research Society International. Osteoarthritis: A Serious Disease, Submitted to the U.S. Food and Drug Administration White Paper (OARSI, 2016).
Sanchez-Lopez, E., Coras, R., Torres, A., Lane, N. E. & Guma, M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 18, 258–275 (2022).
Stephenson, W. et al. Single-cell RNA-seq of rheumatoid arthritis synovial tissue using low-cost microfluidic instrumentation. Nat. Commun. 9, 791 (2018).
Marx, V. Method of the year: spatially resolved transcriptomics. Nat. Methods 18, 9–14 (2021).
Mandelin, A. M. et al. Transcriptional profiling of synovial macrophages using minimally invasive ultrasound-guided synovial biopsies in rheumatoid arthritis. Arthritis Rheumatol. 70, 841–854 (2018).
Kelly, S. et al. Ultrasound-guided synovial biopsy: a safe, well-tolerated and reliable technique for obtaining high-quality synovial tissue from both large and small joints in early arthritis patients. Ann. Rheum. Dis. 74, 611–617 (2015).
Zhang, F. et al. Deconstruction of rheumatoid arthritis synovium defines inflammatory subtypes. Nature 623, 616–624 (2023).
Alivernini, S. et al. Distinct synovial tissue macrophage subsets regulate inflammation and remission in rheumatoid arthritis. Nat. Med. 26, 1295–1306 (2020).
Accelerating Medicines Partnership Rheumatoid Arthritis and Systemic Lupus Erythematosus (AMP RA/SLE) Consortium. et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 20, 928–942 (2019).
Smith, M. H. et al. Drivers of heterogeneity in synovial fibroblasts in rheumatoid arthritis. Nat. Immunol. 24, 1200–1210 (2023).
Mizoguchi, F. et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat. Commun. 9, 789 (2018).
Croft, A. P. et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature 570, 246–251 (2019).
Culemann, S. et al. Locally renewing resident synovial macrophages provide a protective barrier for the joint. Nature 572, 670–675 (2019).
Wood, M. J. et al. Macrophage proliferation distinguishes 2 subgroups of knee osteoarthritis patients. JCI Insight 4, e125325 (2019).
Knights, A. J. et al. Synovial macrophage diversity and activation of M-CSF signaling in post-traumatic osteoarthritis. Preprint at bioRxiv https://doi.org/10.1101/2023.10.03.559514 (2023).
Sebastian, A. et al. Single-cell RNA-Seq reveals changes in immune landscape in post-traumatic osteoarthritis. Front. Immunol. 13, 938075 (2022).
Herrero-Beaumont, G. et al. Systemic osteoarthritis: the difficulty of categorically naming a continuous condition. Aging Clin. Exp. Res. 36, 45 (2024).
McAlindon, T. E. et al. Associations of inflammatory and metabolic biomarkers with incident erosive hand osteoarthritis in the osteoarthritis initiative cohort. Osteoarthr. Cartil. 32, 592–600 (2024).
Wang, Q. et al. Identification of a central role for complement in osteoarthritis. Nat. Med. 17, 1674–1679 (2011).
Silva Santos Ribeiro, P., Willemen, H. L. D. M., Versteeg, S., Martin Gil, C. & Eijkelkamp, N. NLRP3 inflammasome activation in sensory neurons promotes chronic inflammatory and osteoarthritis pain. Immunother. Adv. 3, ltad022 (2023).
Miller, R. J., Malfait, A.-M. & Miller, R. E. The innate immune response as a mediator of osteoarthritis pain. Osteoarthr. Cartil. 28, 562–571 (2020).
Hannani, M. T. et al. From biochemical markers to molecular endotypes of osteoarthritis: a review on validated biomarkers. Expert. Rev. Mol. Diagnostics 24, 23–38 (2024).
Atukorala, I. et al. Synovitis in knee osteoarthritis: a precursor of disease? Ann. Rheum. Dis. 75, 390–395 (2016).
Conaghan, P. G. et al. Clinical and ultrasonographic predictors of joint replacement for knee osteoarthritis: results from a large, 3-year, prospective EULAR study. Ann. Rheum. Dis. 69, 644–647 (2010).
Eitner, A. et al. Pain sensation in human osteoarthritic knee joints is strongly enhanced by diabetes mellitus. Pain 158, 1743–1753 (2017).
Zhang, H. et al. Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Ann. Rheum. Dis. 77, 1524–1534 (2018).
Raghu, H. et al. CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann. Rheum. Dis. 76, 914–922 (2017).
Sakurai, Y. et al. Contribution of synovial macrophages to rat advanced osteoarthritis pain resistant to cyclooxygenase inhibitors. Pain 160, 895–907 (2019).
Blom, A. B. et al. Crucial role of macrophages in matrix metalloproteinase-mediated cartilage destruction during experimental osteoarthritis: involvement of matrix metalloproteinase 3. Arthritis Rheum. 56, 147–157 (2007).
Chou, C.-H. et al. Synovial cell cross-talk with cartilage plays a major role in the pathogenesis of osteoarthritis. Sci. Rep. 10, 10868 (2020).
Wang, C. et al. Transcriptomic profiling of osteoarthritis synovial macrophages reveals a tolerized phenotype compounded by a weak corticosteroid response. Rheumatology 64, 860–869 (2024).
Macchi, V. et al. The infrapatellar fat pad and the synovial membrane: an anatomo‐functional unit. J. Anat. 233, 146–154 (2018).
Tang, S. et al. Single-cell atlas of human infrapatellar fat pad and synovium implicates APOE signaling in osteoarthritis pathology. Sci. Transl. Med. 16, eadf4590 (2024).
South, S. M. et al. Imaging mass cytometry reveals tissue-specific cellular immune phenotypes in the mouse knee following ACL injury. Osteoarthr. Cartil. Open. 5, 100416 (2023).
Peters, H. et al. Cell and transcriptomic diversity of infrapatellar fat pad during knee osteoarthritis. Ann. Rheum. Dis. 84, 351–367 (2025).
Blackler, G. et al. Targeting STAT6-mediated synovial macrophage activation improves pain in experimental knee osteoarthritis. Arthritis Res. Ther. 26, 73 (2024).
Philpott, H. T. et al. Synovial innate immune exhaustion is associated with worse pain in knee osteoarthritis. Arthritis Rheumatol. https://doi.org/10.1002/art.43089 (2024).
Fang, C. et al. TREM2 promotes macrophage polarization from M1 to M2 and suppresses osteoarthritis through the NF-κB/CXCL3 axis. Int. J. Biol. Sci. 20, 1992–2007 (2024).
Raoof, R. et al. Dorsal root ganglia macrophages maintain osteoarthritis pain. J. Neurosci. 41, 8249–8261 (2021).
Martin Gil, C. et al. Myostatin and CXCL11 promote nervous tissue macrophages to maintain osteoarthritis pain. Brain Behav. Immun. 116, 203–215 (2024).
Geraghty, T. et al. Age‐associated changes in knee osteoarthritis, pain‐related behaviors, and dorsal root ganglia immunophenotyping of male and female mice. Arthritis Rheumatol. 75, 1770–1780 (2023).
Domoto, R., Sekiguchi, F., Tsubota, M. & Kawabata, A. Macrophage as a peripheral pain regulator. Cells 10, 1881 (2021).
Nigrovic, P. A. & Lee, D. M. Mast cells in inflammatory arthritis. Arthritis Res. Ther. 7, 1–11 (2005).
Mehta, B. et al. Machine learning identification of thresholds to discriminate osteoarthritis and rheumatoid arthritis synovial inflammation. Arthritis Res. Ther. 25, 31 (2023).
Das, N. et al. Tryptase β regulation of joint lubrication and inflammation via proteoglycan-4 in osteoarthritis. Nat. Commun. 14, 1910 (2023).
Wang, Q. et al. IgE-mediated mast cell activation promotes inflammation and cartilage destruction in osteoarthritis. eLife 8, e39905 (2019).
Baker, M. C., Robinson, W. H. & Ostrom, Q. Genetic association between atopic disease and osteoarthritis. Osteoarthr. Cartil. 32, 220–225 (2024).
Wilkinson, D. J. et al. Matrix metalloproteinase‐13 is fully activated by neutrophil elastase and inactivates its serpin inhibitor, alpha‐1 antitrypsin: implications for osteoarthritis. FEBS J. 289, 121–139 (2022).
Mehrani, Y. et al. The importance of neutrophils in osteoarthritis: current concepts and therapeutic perspectives. Immuno 3, 250–272 (2023).
Luan, T. et al. Identification and analysis of neutrophil extracellular trap-related genes in osteoarthritis by bioinformatics and experimental verification. J. Inflamm. Res. 16, 3837–3852 (2023).
Manukyan, G. et al. Phenotypic and functional characterisation of synovial fluid-derived neutrophils in knee osteoarthritis and knee infection. Osteoarthr. Cartil. 31, 72–82 (2023).
Tchitchek, N. et al. Deep immunophenotyping reveals that autoimmune and autoinflammatory disorders are spread along two immunological axes capturing disease inflammation levels and types. Ann. Rheum. Dis. 83, 638–650 (2024).
Huss, R. S., Huddleston, J. I., Goodman, S. B., Butcher, E. C. & Zabel, B. A. Synovial tissue-infiltrating natural killer cells in osteoarthritis and periprosthetic inflammation: NK cells in degenerative joint disease. Arthritis Rheum. 62, 3799–3805 (2010).
Vivier, E. et al. Innate lymphoid cells: 10 years on. Cell 174, 1054–1066 (2018).
Kraus, V. B. et al. An osteoarthritis pathophysiological continuum revealed by molecular biomarkers. Sci. Adv. 10, eadj6814 (2024).
Loukov, D., Karampatos, S., Maly, M. R. & Bowdish, D. M. E. Monocyte activation is elevated in women with knee-osteoarthritis and associated with inflammation, BMI and pain. Osteoarthr. Cartil. 26, 255–263 (2018).
Zhao, X. et al. CCL3/CCR1 mediates CD14+CD16− circulating monocyte recruitment in knee osteoarthritis progression. Osteoarthr. Cartil. 28, 613–625 (2020).
Dong Kim, K. et al. Adaptive immune cells temper initial innate responses. Nat. Med. 13, 1248–1252 (2007).
Kumar, B. V., Connors, T. J. & Farber, D. L. Human T cell development, localization, and function throughout life. Immunity 48, 202–213 (2018).
Rosshirt, N. et al. Proinflammatory T cell polarization is already present in patients with early knee osteoarthritis. Arthritis Res. Ther. 23, 37 (2021).
Shen, P.-C. et al. T helper cells promote disease progression of osteoarthritis by inducing macrophage inflammatory protein-1γ. Osteoarthr. Cartil. 19, 728–736 (2011).
Nees, T. A. et al. T helper cell infiltration in osteoarthritis-related knee pain and disability. J. Clin. Med. 9, 2423 (2020).
Wheeler, T. A. et al. Mechanical loading of joint modulates T cells in lymph nodes to regulate osteoarthritis. Osteoarthr. Cartil. 32, 287–298 (2024).
Nees, T. A. et al. Infiltration profile of regulatory T cells in osteoarthritis-related pain and disability. Biomedicines 10, 2111 (2022).
Ponchel, F. et al. Changes in peripheral blood immune cell composition in osteoarthritis. Osteoarthr. Cartil. 23, 1870–1878 (2015).
Xie, X., Doody, G. M., Shuweihdi, F., Conaghan, P. G. & Ponchel, F. B-cell capacity for expansion and differentiation into plasma cells are altered in osteoarthritis. Osteoarthr. Cartil. 31, 1176–1188 (2023).
Platzer, H. et al. CD8+ T cells in OA knee joints are differentiated into subsets depending on OA stage and compartment. J. Clin. Med. 11, 2814 (2022).
Trajerova, M. et al. Knee osteoarthritis phenotypes based on synovial fluid immune cells correlate with clinical outcome trajectories. Osteoarthr. Cartil. 30, 1583–1592 (2022).
Burt, K. G. & Scanzello, C. R. B cells in osteoarthritis: simply a sign or a target for therapy? Osteoarthr. Cartil. 31, 1148–1151 (2023).
Sun, H. et al. IgM+CD27+ B cells possessed regulatory function and represented the main source of B cell-derived IL-10 in the synovial fluid of osteoarthritis patients. Hum. Immunol. 80, 263–269 (2019).
Kapoor, M., Martel-Pelletier, J., Lajeunesse, D., Pelletier, J.-P. & Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 7, 33–42 (2011).
Xie, X. et al. Auto-antibodies to post-translationally modified proteins in osteoarthritis. Osteoarthr. Cartil. 29, 924–933 (2021).
Pacquelet, S. et al. Interleukin 17, a nitric oxide-producing cytokine with a peroxynitrite-independent inhibitory effect on proteoglycan synthesis. J. Rheumatol. 29, 2602–2610 (2002).
Sun, W. et al. IL-17A exacerbates synovial inflammation in osteoarthritis via activation of endoplasmic reticulum stress. Int. Immunopharmacol. 145, 113733 (2025).
Sinkeviciute, D., Aspberg, A., He, Y., Bay-Jensen, A.-C. & Önnerfjord, P. Characterization of the interleukin-17 effect on articular cartilage in a translational model: an explorative study. BMC Rheumatol. 4, 30 (2020).
Wisniewska, E. et al. Infrapatellar fat pad modulates osteoarthritis-associated cytokine and MMP expression in human articular chondrocytes. Cells 12, 2850 (2023).
Zapata-Linares, N. et al. Systemic and joint adipose tissue lipids and their role in osteoarthritis. Biochimie 227, 130–138 (2024).
Apinun, J. et al. Immune mediators in osteoarthritis: infrapatellar fat pad-infiltrating CD8+ T cells are increased in osteoarthritic patients with higher clinical radiographic grading. Int. J. Rheumatol. 2016, 9525724 (2016).
Xu, C. et al. Causal associations between circulating immune cells and osteoarthritis: a bidirectional Mendelian randomization study. Int. Immunopharmacol. 142, 113156 (2024).
Zhu, W. et al. Alterations in peripheral T cell and B cell subsets in patients with osteoarthritis. Clin. Rheumatol. 39, 523–532 (2020).
Binvignat, M. et al. Immunological profiling in knee osteoarthritis: Treg dysfunction as key driver of pain. Preprint at bioRxiv https://doi.org/10.1101/2024.10.12.618016 (2024).
Nakamura, H., Yoshino, S., Kato, T., Tsuruha, J. & Nishioka, K. T-cell mediated inflammatory pathway in osteoarthritis. Osteoarthr. Cartil. 7, 401–402 (1999).
Kim, H.-Y. et al. Enhanced T cell proliferative response to type II collagen and synthetic peptide CII (255–274) in patients with rheumatoid arthritis. Arthritis Rheum. 42, 2085–2093 (1999).
Tsuruha, J.-I. et al. Autoimmunity against YKL-39, a human cartilage derived protein, in patients with osteoarthritis. J. Rheumatol. 29, 1459–1466 (2002).
Sakata, M. et al. Osteoarthritic articular chondrocytes stimulate autologous T cell responses in vitro. Clin. Exp. Rheumatol. 21, 704–710 (2003).
De Jong, H. et al. Cartilage proteoglycan aggrecan epitopes induce proinflammatory autoreactive T-cell responses in rheumatoid arthritis and osteoarthritis. Ann. Rheum. Dis. 69, 255–262 (2010).
Camacho-Encina, M. et al. Discovery of an autoantibody signature for the early diagnosis of knee osteoarthritis: data from the Osteoarthritis Initiative. Ann. Rheum. Dis. 78, 1699–1705 (2019).
Boutet, M.-A. et al. Comparative analysis of late-stage rheumatoid arthritis and osteoarthritis reveals shared histopathological features. Osteoarthr. Cartil. 32, 166–176 (2024).
Favero, M. et al. Infrapatellar fat pad features in osteoarthritis: a histopathological and molecular study. Rheumatology 56, 1784–1793 (2017).
Marshall, D. A. et al. Existing comorbidities in people with osteoarthritis: a retrospective analysis of a population-based cohort in Alberta, Canada. BMJ Open. 9, e033334 (2019).
Visser, A. W. et al. The relative contribution of mechanical stress and systemic processes in different types of osteoarthritis: the NEO study. Ann. Rheum. Dis. 74, 1842–1847 (2015).
Jin, X. et al. Circulating C reactive protein in osteoarthritis: a systematic review and meta-analysis. Ann. Rheum. Dis. 74, 703–710 (2015).
Attur, M. et al. Plasma levels of interleukin-1 receptor antagonist (IL1Ra) predict radiographic progression of symptomatic knee osteoarthritis. Osteoarthr. Cartil. 23, 1915–1924 (2015).
Hotamisligil, G. S. Inflammation, metaflammation and immunometabolic disorders. Nature 542, 177–185 (2017).
Fulop, T. et al. Immunology of aging: the birth of inflammaging. Clin. Rev. Allerg. Immunol. 64, 109–122 (2021).
Courties, A., Berenbaum, F. & Sellam, J. The phenotypic approach to osteoarthritis: a look at metabolic syndrome-associated osteoarthritis. Jt Bone Spine 86, 725–730 (2019).
Binvignat, M., Sellam, J., Berenbaum, F. & Felson, D. T. The role of obesity and adipose tissue dysfunction in osteoarthritis pain. Nat. Rev. Rheumatol. 20, 565–584 (2024).
Unamuno, X. et al. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur. J. Clin. Invest. 48, e12997 (2018).
Francisco, V. et al. Adipokines: linking metabolic syndrome, the immune system, and arthritic diseases. Biochem. Pharmacol. 165, 196–206 (2019).
Frühbeck, G., Catalán, V., Rodríguez, A. & Gómez-Ambrosi, J. Adiponectin-leptin ratio: a promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte 7, 57–62 (2018).
Sellam, J. et al. Pain in women with knee and/or hip osteoarthritis is related to systemic inflammation and to adipose tissue dysfunction: cross-sectional results of the KHOALA cohort. Semin. Arthritis Rheum. 51, 129–136 (2021).
De Roover, A., Escribano-Núñez, A., Monteagudo, S. & Lories, R. Fundamentals of osteoarthritis: inflammatory mediators in osteoarthritis. Osteoarthr. Cartil. 31, 1303–1311 (2023).
Swindell, W. R. et al. Robust shifts in S100a9 expression with aging: a novel mechanism for chronic inflammation. Sci. Rep. 3, 1215 (2013).
Greene, M. A. & Loeser, R. F. Aging-related inflammation in osteoarthritis. Osteoarthr. Cartil. 23, 1966–1971 (2015).
Liu, Z. et al. Immunosenescence: molecular mechanisms and diseases. Sig. Transduct. Target. Ther. 8, 200 (2023).
Khan, S. et al. B cells promote T cell immunosenescence and mammalian aging parameters. Preprint at bioRxiv https://doi.org/10.1101/2023.09.12.556363 (2023).
Han, Z., Wang, K., Ding, S. & Zhang, M. Cross-talk of inflammation and cellular senescence: a new insight into the occurrence and progression of osteoarthritis. Bone Res. 12, 69 (2024).
Li, H., Liu, W. & Xie, J. Circulating interleukin-6 levels and cardiovascular and all-cause mortality in the elderly population: a meta-analysis. Arch. Gerontol. Geriatr. 73, 257–262 (2017).
Forcina, L., Franceschi, C. & Musarò, A. The hormetic and hermetic role of IL-6. Ageing Res. Rev. 80, 101697 (2022).
Widjaja, A. A. et al. Inhibition of IL-11 signalling extends mammalian healthspan and lifespan. Nature 632, 157–165 (2024).
Binvignat, M., Sokol, H., Mariotti-Ferrandiz, E., Berenbaum, F. & Sellam, J. Osteoarthritis and gut microbiome. Jt Bone Spine 88, 105203 (2021).
Marietta, E., Rishi, A. & Taneja, V. Immunogenetic control of the intestinal microbiota. Immunology 145, 313–322 (2015).
Guan, Z. et al. Gut microbiome dysbiosis alleviates the progression of osteoarthritis in mice. Clin. Sci. 134, 3159–3174 (2020).
Ulici, V. et al. Osteoarthritis induced by destabilization of the medial meniscus is reduced in germ-free mice. Osteoarthr. Cartil. 26, 1098–1109 (2018).
Schott, E. M. et al. Targeting the gut microbiome to treat the osteoarthritis of obesity. JCI Insight 3, e95997 (2018).
Huang, Z. Y., Stabler, T., Pei, F. X. & Kraus, V. B. Both systemic and local lipopolysaccharide (LPS) burden are associated with knee OA severity and inflammation. Osteoarthr. Cartil. 24, 1769–1775 (2016).
Boer, C. G. et al. Intestinal microbiome composition and its relation to joint pain and inflammation. Nat. Commun. 10, 4881 (2019).
Dunn, C. M. et al. Identification of cartilage microbial DNA signatures and associations with knee and hip osteoarthritis. Arthritis Rheumatol. 72, 1111–1122 (2020).
Huang, Z. Y. et al. Biomarkers of inflammation — LBP and TLR — predict progression of knee osteoarthritis in the DOXY clinical trial. Osteoarthr. Cartil. 26, 1658–1665 (2018).
Wei, J. et al. Association between gut microbiota and symptomatic hand osteoarthritis: data from the Xiangya Osteoarthritis Study. Arthritis Rheumatol. 73, 1656–1662 (2021).
Binvignat, M. et al. Serum tryptophan metabolites are associated with erosive hand osteoarthritis and pain: results from the DIGICOD cohort. Osteoarthr. Cartil. 31, 1132–1143 (2023).
Binvignat, M. et al. Serum intestinal permeability biomarkers are associated with erosive hand osteoarthritis and radiographic severity: results from the DIGICOD cohort. Osteoarthr. Cartil. 32, 747 (2024).
Moulin, D. et al. Counteracting tryptophan metabolism alterations as a new therapeutic strategy for rheumatoid arthritis. Ann. Rheum. Dis. 83, 312–323 (2024).
Cho, K.-H. et al. Lactobacillus (LA-1) and butyrate inhibit osteoarthritis by controlling autophagy and inflammatory cell death of chondrocytes. Front. Immunol. 13, 930511 (2022).
Huang, Z. et al. Faecal microbiota transplantation from metabolically compromised human donors accelerates osteoarthritis in mice. Ann. Rheum. Dis. 79, 646–656 (2020).
Kamps, A. et al. Occurrence of comorbidity following osteoarthritis diagnosis: a cohort study in the Netherlands. Osteoarthr. Cartil. 31, 519–528 (2023).
Magnusson, K., Turkiewicz, A., Dell’Isola, A. & Englund, M. Shared genetic factors between osteoarthritis and cardiovascular disease may underlie common etiology. Nat. Commun. 15, 9569 (2024).
Weber, A. et al. Association between osteoarthritis and increased risk of dementia: a systemic review and meta-analysis. Medicine 98, e14355 (2019).
Kyrkanides, S. et al. Osteoarthritis accelerates and exacerbates Alzheimer’s disease pathology in mice. J. Neuroinflammation 8, 112 (2011).
Favero, M. et al. Erosive hand osteoarthritis: latest findings and outlook. Nat. Rev. Rheumatol. 18, 171–183 (2022).
Kloppenburg, M. et al. Etanercept in patients with inflammatory hand osteoarthritis (EHOA): a multicentre, randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 77, 1757–1764 (2018).
Chevalier, X. et al. Adalimumab in patients with hand osteoarthritis refractory to analgesics and NSAIDs: a randomised, multicentre, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 74, 1697–1705 (2015).
Richette, P. et al. Efficacy of tocilizumab in patients with hand osteoarthritis: double blind, randomised, placebo-controlled, multicentre trial. Ann. Rheum. Dis. 80, 349–355 (2021).
Schett, G. et al. Anti-granulocyte-macrophage colony-stimulating factor antibody otilimab in patients with hand osteoarthritis: a phase 2a randomised trial. Lancet Rheumatol. 2, e623–e632 (2020).
Fleischmann, R. M. et al. A phase II trial of lutikizumab, an anti-interleukin‐1α/β dual variable domain immunoglobulin, in knee osteoarthritis patients with synovitis. Arthritis Rheumatol. 71, 1056–1069 (2019).
Kloppenburg, M. et al. Phase IIa, placebo-controlled, randomised study of lutikizumab, an anti-interleukin-1α and anti-interleukin-1β dual variable domain immunoglobulin, in patients with erosive hand osteoarthritis. Ann. Rheum. Dis. 78, 413–420 (2019).
Schieker, M. et al. Effects of interleukin-1β inhibition on incident hip and knee replacement: exploratory analyses from a randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 173, 509–515 (2020).
Wittoek, R., Verbruggen, G., Vanhaverbeke, T., Colman, R. & Elewaut, D. RANKL blockade for erosive hand osteoarthritis: a randomized placebo-controlled phase 2a trial. Nat. Med. 30, 829–836 (2024).
Kingsbury, S. R. et al. Pain reduction with oral methotrexate in knee osteoarthritis: a randomized, placebo-controlled clinical trial. Ann. Intern. Med. 177, 1145–1156 (2024).
Wang, Y. et al. Methotrexate to treat hand osteoarthritis with synovitis (METHODS): an Australian, multisite, parallel-group, double-blind, randomised, placebo-controlled trial. Lancet 402, 1764–1772 (2023).
Ferrero, S. et al. Methotrexate treatment in hand osteoarthritis refractory to usual treatments: a randomised, double-blind, placebo-controlled trial. Semin. Arthritis Rheum. 51, 831–838 (2021).
Najm, A., Alunno, A., Gwinnutt, J. M., Weill, C. & Berenbaum, F. Efficacy of intra-articular corticosteroid injections in knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Jt Bone Spine 88, 105198 (2021).
Guermazi, A., Hunter, D. J. & Kloppenburg, M. Debate: intra-articular steroid injections for osteoarthritis — harmful or helpful? Osteoarthr. Imaging 3, 100163 (2023).
Kroon, F. P. B. et al. Results of a 6-week treatment with 10 mg prednisolone in patients with hand osteoarthritis (HOPE): a double-blind, randomised, placebo-controlled trial. Lancet 394, 1993–2001 (2019).
Døssing, A. et al. Colchicine twice a day for hand osteoarthritis (COLOR): a double-blind, randomised, placebo-controlled trial. Lancet Rheumatol. 5, e254–e262 (2023).
Heijman, M. W. J. et al. Association of low-dose colchicine with incidence of knee and hip replacements: exploratory analyses from a randomized, controlled, double-blind trial. Ann. Intern. Med. 176, 737–742 (2023).
Kingsbury, S. R. et al. Hydroxychloroquine effectiveness in reducing symptoms of hand osteoarthritis: a randomized trial. Ann. Intern. Med. 168, 385 (2018).
Wang, Y. et al. Effect of atorvastatin on knee cartilage volume in patients with symptomatic knee osteoarthritis: results from a randomized placebo‐controlled trial. Arthritis Rheumatol. 73, 2035–2043 (2021).
Grigsby, E. et al. XT-150 — a novel immunomodulatory gene therapy for osteoarthritis pain in phase 2b development. Osteoarthr. Cartil. 29, S12 (2021).
Meurot, C. et al. Liraglutide, a glucagon-like peptide 1 receptor agonist, exerts analgesic, anti-inflammatory and anti-degradative actions in osteoarthritis. Sci. Rep. 12, 1567 (2022).
Remst, D. F. G. et al. Gene expression analysis of murine and human osteoarthritis synovium reveals elevation of transforming growth factor β-responsive genes in osteoarthritis‐related fibrosis. Arthritis Rheumatol. 66, 647–656 (2014).
Yan, J. et al. Nintedanib ameliorates osteoarthritis in mice by inhibiting synovial inflammation and fibrosis caused by M1 polarization of synovial macrophages via the MAPK / PI3K-AKT pathway. FASEB J. 37, e23177 (2023).
Pers, Y.-M. et al. Recommendations from the French Societies of Rheumatology and Physical Medicine and Rehabilitation on the non-pharmacological management of knee osteoarthritis. Ann. Phys. Rehabil. Med. 67, 101883 (2024).
Walrabenstein, W. et al. A multidisciplinary lifestyle program for metabolic syndrome-associated osteoarthritis: the ‘Plants for Joints’ randomized controlled trial. Osteoarthr. Cartil. 31, 1491–1500 (2023).
Mobasheri, A. & Loeser, R. Clinical phenotypes, molecular endotypes and theratypes in OA therapeutic development. Nat. Rev. Rheumatol. 20, 525–526 (2024).
Angelini, F. et al. Osteoarthritis endotype discovery via clustering of biochemical marker data. Ann. Rheum. Dis. 81, 666–675 (2022).
Siaton, B. C., Hogans, B. H. & Hochberg, M. C. Precision medicine in osteoarthritis: not yet ready for prime time. Expert. Rev. Precis. Med. Drug. Dev. 6, 5–8 (2021).
Barry, F. & Murphy, M. Mesenchymal stem cells in joint disease and repair. Nat. Rev. Rheumatol. 9, 584–594 (2013).
Ankrum, J. A., Ong, J. F. & Karp, J. M. Mesenchymal stem cells: immune evasive, not immune privileged. Nat. Biotechnol. 32, 252–260 (2014).
Moll, G. et al. Mesenchymal stromal cells engage complement and complement receptor bearing innate effector cells to modulate immune responses. PLoS ONE 6, e21703 (2011).
Chen, C.-F. et al. Treatment of knee osteoarthritis with intra-articular injection of allogeneic adipose-derived stem cells (ADSCs) ELIXCYTE®: a phase I/II, randomized, active-control, single-blind, multiple-center clinical trial. Stem Cell Res. Ther. 12, 562 (2021).
Bertolino, G. M., Maumus, M., Jorgensen, C. & Noël, D. Therapeutic potential in rheumatic diseases of extracellular vesicles derived from mesenchymal stromal cells. Nat. Rev. Rheumatol. 19, 682–694 (2023).
Najar, M. et al. Mesenchymal stromal cells and immunomodulation: a gathering of regulatory immune cells. Cytotherapy 18, 160–171 (2016).
Wang, Y., Fang, J., Liu, B., Shao, C. & Shi, Y. Reciprocal regulation of mesenchymal stem cells and immune responses. Cell Stem Cell 29, 1515–1530 (2022).
Le Blanc, K. et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371, 1579–1586 (2008).
Giri, J., Das, R., Nylen, E., Chinnadurai, R. & Galipeau, J. CCL2 and CXCL12 derived from mesenchymal stromal cells cooperatively polarize IL-10+ tissue macrophages to mitigate gut injury. Cell Rep. 30, 1923–1934.e4 (2020).
Chiesa, S. et al. Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proc. Natl Acad. Sci. USA 108, 17384–17389 (2011).
Abbasi, B., Shamsasenjan, K., Ahmadi, M., Beheshti, S. A. & Saleh, M. Mesenchymal stem cells and natural killer cells interaction mechanisms and potential clinical applications. Stem Cell Res. Ther. 13, 97 (2022).
Glennie, S., Soeiro, I., Dyson, P. J., Lam, E. W.-F. & Dazzi, F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 105, 2821–2827 (2005).
Hagmann, S. et al. The influence of bone marrow- and synovium-derived mesenchymal stromal cells from osteoarthritis patients on regulatory T cells in co-culture. Clin. Exp. Immunol. 173, 454–462 (2013).
Corcione, A. et al. Human mesenchymal stem cells modulate B-cell functions. Blood 107, 367–372 (2006).
Copp, G., Robb, K. P. & Viswanathan, S. Culture-expanded mesenchymal stromal cell therapy: does it work in knee osteoarthritis? A pathway to clinical success. Cell Mol. Immunol. 20, 626–650 (2023).
Mautner, K. et al. Cell-based versus corticosteroid injections for knee pain in osteoarthritis: a randomized phase 3 trial. Nat. Med. 29, 3120–3126 (2023).
Lagneau, N. et al. Harnessing cell-material interactions to control stem cell secretion for osteoarthritis treatment. Biomaterials 296, 122091 (2023).
Cherian, J. J. et al. Preliminary results of a phase II randomized study to determine the efficacy and safety of genetically engineered allogeneic human chondrocytes expressing TGF-β1 in patients with grade 3 chronic degenerative joint disease of the knee. Osteoarthr. Cartil. 23, 2109–2118 (2015).
Chuang, C.-H. et al. Enriched peripheral blood-derived mononuclear cells for treating knee osteoarthritis. Cell Transpl. 32, 09636897221149445 (2023).
Liang, C. et al. Engineered M2a macrophages for the treatment of osteoarthritis. Front. Immunol. 13, 1054938 (2022).
Song, X. et al. An injectable thermosensitive hydrogel delivering M2 macrophage-derived exosomes alleviates osteoarthritis by promoting synovial lymphangiogenesis. Acta Biomater. 189, 130–142 (2024).
Ma, Y. et al. Artificial M2 macrophages for disease-modifying osteoarthritis therapeutics. Biomaterials 274, 120865 (2021).
Craft, A. M. et al. Generation of articular chondrocytes from human pluripotent stem cells. Nat. Biotechnol. 33, 638–645 (2015).
Tiwari, S. K., Wong, W. J., Moreira, M., Pasqualini, C. & Ginhoux, F. Induced pluripotent stem cell-derived macrophages as a platform for modelling human disease. Nat. Rev. Immunol. 25, 108–124 (2025).
Kelly, K. et al. Two-year safety outcomes of iPS cell-derived mesenchymal stromal cells in acute steroid-resistant graft-versus-host disease. Nat. Med. 30, 1556–1558 (2024).
Byron, S. A., Van Keuren-Jensen, K. R., Engelthaler, D. M., Carpten, J. D. & Craig, D. W. Translating RNA sequencing into clinical diagnostics: opportunities and challenges. Nat. Rev. Genet. 17, 257–271 (2016).
Mobasheri, A. et al. Recent advances in understanding the phenotypes of osteoarthritis. F1000Res 8, 2091 (2019).
Acknowledgements
We particularly would like to thank A. Cardon and C. Morizot for their involvement in the preparation of the Review outline and for sharing their suggestions related to the preparation of this manuscript. We would also like to thank the following funding sources for their support: Fondation pour la recherche médicale (grant number ARF202004011786 to M.-A.B.; EQU202303016276 to J.G. and M.-A.B. and PFG-P 2022 to J.G.), Inserm (ATIP-Avenir program to M.-A.B.), Region Grand Est (FRCR TARGET to D.M.), Agence Nationale de la Recherche (ANR-18-CE18-0010 PPAROA to J.G., ANR-23-CE18-0018 TrypENGINE to D.M.), Fondation Arthritis (Projet labellisé TRYPTHERA to D.M.; and Projet émergent SPOTT to M.-A.B.), Going Inside Osteoarthritis-Related Pain Phenotyping (GO-PAIN) ERA-NET NEURON grant (to J.S.) and Pfizer ADVANCE 2020 grant (to J.S. and F.B.).
Author information
Authors and Affiliations
Contributions
M.-A.B., D.M., J.G., J.S., and F.B. researched data for the article and wrote the article. All authors contributed substantially to discussion of the content and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
F.B. received an institutional grant from TRB Chemedica and Pfizer and consulting fees from AstraZeneca, Boehringer Ingelheim, Bone Therapeutics, Cellprothera, Galapagos, Gilead, Grunenthal, GSK, Lilly, MerckSerono, MSD, Nordic Bioscience, Novartis, Pfizer, Roche, Sandoz, Sanofi, Servier, UCB, Peptinov, 4 P Pharma and 4Moving Biotech. J.S. reports personal fees from MSD, Pfizer, AbbVie, Fresenius Kabi, BMS, Lilly, Novartis, Galapagos, AstraZeneca, UCB, Grunenthal and Janssen and research grants from Pfizer and Schwa Medico. J.G. received consulting fees from BMS, Graftys, PKmed, HTL biotechnology, Cellprothera, GSK, Peptinov and CEVA.
Peer review
Peer review information
Nature Reviews Rheumatology thanks Mary Goldring; Mohit Kapoor, who co-reviewed with Jason Rockel and Yusheng Li for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria
A search of PubMed for original relevant and high-quality articles published between 2005 and 2024 (a few older and original studies are also cited in the text where relevant) that focus on the diversity and role of innate and adaptive immune cells in OA. Search terms included “OSTEOARTHRITIS”, “IMMUNE CELLS”, “IMMUNITY”, “MICROBIOTA” and “TREATMENT”, alone and in combination. All articles identified were English-language, full-text papers. We also searched the reference lists of identified articles for additional relevant papers.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moulin, D., Sellam, J., Berenbaum, F. et al. The role of the immune system in osteoarthritis: mechanisms, challenges and future directions. Nat Rev Rheumatol 21, 221–236 (2025). https://doi.org/10.1038/s41584-025-01223-y
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41584-025-01223-y
This article is cited by
-
Synovial transcriptome analysis reveals ShenTong ZhuYu Tang alleviates osteoarthritis by targeting MMP-9 and immune microenvironment
Journal of Orthopaedic Surgery and Research (2026)
-
Identification and validation of immune-related biomarkers in osteoarthritis via integrated bioinformatics and experimental approaches
Cytotechnology (2026)
-
Methodological concerns and mechanistic gaps in the association between unhealthy lifestyle and comorbid type 2 diabetes and arthritis
Aging Clinical and Experimental Research (2026)
-
Mitochondrial-targeted ROS scavenger delivery using selenium-doped layered double hydroxide nanocomposites for enhanced osteoarthritis therapy via inflammation suppression and macrophage metabolism reprogramming
Advanced Composites and Hybrid Materials (2026)
-
Pathogenesis and therapeutic strategies of osteoarthritis: roles of immune cells, inflammatory mediators, and pathogenic signaling pathways
Immunity & Inflammation (2026)