Abstract
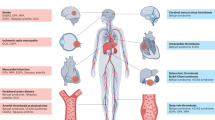
Many monogenic autoinflammatory diseases, including DADA2 (deficiency of adenosine deaminase 2), HA20 (haploinsufficiency of A20), SAVI (STING-associated vasculopathy with onset in infancy), COPA syndrome, LAVLI (LYN kinase-associated vasculopathy and liver fibrosis) and VEXAS (vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic) syndrome, present predominantly with vasculitis and constitute a substantial subgroup of vasculitic conditions associated with a ‘probable aetiology’. The spectrum of monogenic vasculitis encompasses all sizes and types of blood vessel, ranging from large vessels to medium-size and small vessels, and from the arterial side to the venous side of the vasculature. Monogenic vasculitis typically starts early in life during infancy or childhood; VEXAS syndrome, which presents in late adulthood, is an exception. The activation of myeloid cells via inflammasome and nuclear factor-κB pathways, type I interferon-enhanced autoimmune mechanisms and/or dysregulated adaptive immune responses have an important role in the development of immune-mediated endothelial dysfunction and vascular damage. Genetic testing is essential for the diagnosis of underlying monogenic autoinflammatory diseases; however, the penetrance of genetic variants can vary. Increased awareness and recognition of distinctive clinical findings could facilitate earlier diagnosis and allow for more-targeted treatments.
Key points
-
Monogenic vasculitis usually starts during early childhood, whereas VEXAS (vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic) syndrome typically develops in late adulthood.
-
Early recognition of distinctive clinical findings (such as early strokes) might aid diagnosis.
-
Genetic diagnosis is crucial, but variable penetrance of variants should be kept in mind when interpreting findings and during genetic counselling.
-
Activation of myeloid cells, type I interferon-enhanced autoimmune responses and endothelial dysfunction contribute to vascular damage.
-
Increased awareness of these rare diseases could aid earlier diagnosis and initiation of targeted treatments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Zhou, Q. et al. Early-onset stroke and vasculopathy associated with mutations in ADA2. N. Engl. J. Med. 370, 911–920 (2014).
Navon Elkan, P. et al. Mutant adenosine deaminase 2 in a polyarteritis nodosa vasculopathy. N. Engl. J. Med. 370, 921–931 (2014).
Zhou, Q. et al. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat. Genet. 48, 67–73 (2016).
Liu, Y. et al. Activated STING in a vascular and pulmonary syndrome. N. Engl. J. Med. 371, 507–518 (2014).
de Jesus, A. A. et al. Constitutively active Lyn kinase causes a cutaneous small vessel vasculitis and liver fibrosis syndrome. Nat. Commun. 14, 1502 (2023).
Beck, D. B. et al. Somatic mutations in UBA1 and severe adult-onset autoinflammatory disease. N. Engl. J. Med. 383, 2628–2638 (2020).
Abbara, S., Grateau, G., Ducharme-Benard, S., Saadoun, D. & Georgin-Lavialle, S. Association of vasculitis and familial mediterranean fever. Front. Immunol. 10, 763 (2019).
Balci-Peynircioglu, B. et al. Comorbidities in familial Mediterranean fever: analysis of 2000 genetically confirmed patients. Rheumatology 59, 1372–1380 (2020).
Seyahi, E., Ugurlu, S., Amikishiyev, S. & Gul, A. Behcet disease, familial Mediterranean fever and MEFV variations: more than just an association. Clin. Immunol. 251, 109630 (2023).
Ozdogan, H. et al. Vasculitis in familial Mediterranean fever. J. Rheumatol. 24, 323–327 (1997).
Kahr, W. H. et al. Loss of the Arp2/3 complex component ARPC1B causes platelet abnormalities and predisposes to inflammatory disease. Nat. Commun. 8, 14816 (2017).
Kuijpers, T. W. et al. Combined immunodeficiency with severe inflammation and allergy caused by ARPC1B deficiency. J. Allergy Clin. Immunol. 140, 273–277 e210 (2017).
Burleigh, A. et al. Genetic testing of Behcet’s disease using next-generation sequencing to identify monogenic mimics and HLA-B*51. Rheumatology 63, 3457–3470 (2023).
Kone-Paut, I., Sanchez, E., Le Quellec, A., Manna, R. & Touitou, I. Autoinflammatory gene mutations in Behcet’s disease. Ann. Rheum. Dis. 66, 832–834 (2007).
Thors, V. S. et al. Periodic fever in MVK deficiency: a patient initially diagnosed with incomplete Kawasaki disease. Pediatrics 133, e461–e465 (2014).
De Pieri, C. et al. Different presentations of mevalonate kinase deficiency: a case series. Clin. Exp. Rheumatol. 33, 437–442 (2015).
Kusne, Y. et al. Venous and arterial thrombosis in patients with VEXAS syndrome. Blood 143, 2190–2200 (2024).
Watanabe, R., Kiji, M. & Hashimoto, M. Vasculitis associated with VEXAS syndrome: a literature review. Front. Med. 9, 983939 (2022).
Jennette, J. C. et al. 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Arthritis Rheum. 65, 1–11 (2013).
Ozen, S. et al. A monogenic disease with a variety of phenotypes: deficiency of adenosine deaminase 2. J. Rheumatol. 47, 117–125 (2020).
Meyts, I. & Aksentijevich, I. Deficiency of adenosine deaminase 2 (DADA2): updates on the phenotype, genetics, pathogenesis, and treatment. J. Clin. Immunol. 38, 569–578 (2018).
Lee, P. Y. et al. Evaluation and management of deficiency of adenosine deaminase 2: an international consensus statement. JAMA Netw. Open 6, e2315894 (2023).
Kasap Cuceoglu, M. et al. Systematic review of childhood-onset polyarteritis nodosa and DADA2. Semin. Arthritis Rheum. 51, 559–564 (2021).
Lee, P. Y., Aksentijevich, I. & Zhou, Q. Mechanisms of vascular inflammation in deficiency of adenosine deaminase 2 (DADA2). Semin. Immunopathol. 44, 269–280 (2022).
Schepp, J. et al. Screening of 181 patients with antibody deficiency for deficiency of adenosine deaminase 2 sheds new light on the disease in adulthood. Arthritis Rheumatol. 69, 1689–1700 (2017).
Signa, S. et al. Adenosine deaminase 2 deficiency (DADA2): a crosstalk between innate and adaptive immunity. Front. Immunol. 13, 935957 (2022).
Belot, A. et al. Mutations in CECR1 associated with a neutrophil signature in peripheral blood. Pediatr. Rheumatol. Online J. 12, 44 (2014).
Carmona-Rivera, C. et al. Deficiency of adenosine deaminase 2 triggers adenosine-mediated NETosis and TNF production in patients with DADA2. Blood 134, 395–406 (2019).
Brix, A. et al. ADA2 regulates inflammation and hematopoietic stem cell emergence via the A(2b)R pathway in zebrafish. Commun. Biol. 7, 615 (2024).
Aksentijevich, I., Sampaio Moura, N. & Barron, K. Adenosine deaminase 2 deficiency. GeneReviews https://www.ncbi.nlm.nih.gov/books/NBK544951/ (2019).
Grossi, A. et al. ADA2 deficiency due to a novel structural variation in 22q11.1. Clin. Genet. 95, 732–733 (2019).
Jee, H. et al. Comprehensive analysis of ADA2 genetic variants and estimation of carrier frequency driven by a function-based approach. J. Allergy Clin. Immunol. 149, 379–387 (2022).
Lee, P. Y. et al. Genotype and functional correlates of disease phenotype in deficiency of adenosine deaminase 2 (DADA2). J. Allergy Clin. Immunol. 145, 1664–1672 e1610 (2020).
Cooray, S. et al. Anti-tumour necrosis factor treatment for the prevention of ischaemic events in patients with deficiency of adenosine deaminase 2 (DADA2). Rheumatology 60, 4373–4378 (2021).
Ombrello, A. K. et al. Treatment strategies for deficiency of adenosine deaminase 2. N. Engl. J. Med. 380, 1582–1584 (2019).
Zoccolillo, M. et al. Lentiviral correction of enzymatic activity restrains macrophage inflammation in adenosine deaminase 2 deficiency. Blood Adv. 5, 3174–3187 (2021).
Aeschlimann, F. A. et al. A20 haploinsufficiency (HA20): clinical phenotypes and disease course of patients with a newly recognised NF-kB-mediated autoinflammatory disease. Ann. Rheum. Dis. 77, 728–735 (2018).
Karri, U., Harasimowicz, M., Carpio Tumba, M. & Schwartz, D. M. The complexity of being a20: from biological functions to genetic associations. J. Clin. Immunol. 44, 76 (2024).
Elhani, I. et al. A20 haploinsufficiency: a systematic review of 177 cases. J. Invest. Dermatol. 144, 1282–1294 e1288 (2024).
Gans, M. D., Wang, H., Moura, N. S., Aksentijevich, I. & Rubinstein, A. A20 haploinsufficiency presenting with a combined immunodeficiency. J. Clin. Immunol. 40, 1041–1044 (2020).
Bettiol, A. et al. Vascular Behcet syndrome: from pathogenesis to treatment. Nat. Rev. Rheumatol. 19, 111–126 (2023).
Niwano, T. et al. An adult case of suspected A20 haploinsufficiency mimicking polyarteritis nodosa. Rheumatology 61, e337–e340 (2022).
Kadowaki, T., Kadowaki, S. & Ohnishi, H. A20 haploinsufficiency in East Asia. Front. Immunol. 12, 780689 (2021).
Ma, A. & Malynn, B. A. A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nat. Rev. Immunol. 12, 774–785 (2012).
Martens, A. & van Loo, G. A20 at the crossroads of cell death, inflammation, and autoimmunity. Cold Spring Harb. Perspect. Biol. 12, a036418 (2020).
Martens, A. et al. Two distinct ubiquitin-binding motifs in A20 mediate its anti-inflammatory and cell-protective activities. Nat. Immunol. 21, 381–387 (2020).
Razani, B. et al. Non-catalytic ubiquitin binding by A20 prevents psoriatic arthritis-like disease and inflammation. Nat. Immunol. 21, 422–433 (2020).
Duncan, C. J. A. et al. Early-onset autoimmune disease due to a heterozygous loss-of-function mutation in TNFAIP3 (A20). Ann. Rheum. Dis. 77, 783–786 (2018).
Lin, B. & Goldbach-Mansky, R. Pathogenic insights from genetic causes of autoinflammatory inflammasomopathies and interferonopathies. J. Allergy Clin. Immunol. 149, 819–832 (2022).
Hofer, M. J. et al. The prototypical interferonopathy: Aicardi-Goutieres syndrome from bedside to bench. Immunol. Rev. 327, 83–99 (2024).
Jeremiah, N. et al. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J. Clin. Invest. 124, 5516–5520 (2014).
Fremond, M. L. & Crow, Y. J. STING-mediated lung inflammation and beyond. J. Clin. Immunol. 41, 501–514 (2021).
Clarke, S. L. N. et al. Type 1 interferonopathy presenting as juvenile idiopathic arthritis with interstitial lung disease: report of a new phenotype. Pediatr. Rheumatol. Online J. 18, 37 (2020).
Anjani, G. et al. Deforming polyarthritis in a North Indian family-clinical expansion of STING-associated vasculopathy with onset in infancy (SAVI). J. Clin. Immunol. 41, 209–211 (2021).
Staels, F. et al. Adult-onset ANCA-associated vasculitis in SAVI: extension of the phenotypic spectrum, case report and review of the literature. Front. Immunol. 11, 575219 (2020).
Picard, C. et al. Severe pulmonary fibrosis as the first manifestation of interferonopathy (TMEM173 Mutation). Chest 150, e65–e71 (2016).
Konig, N. et al. Familial chilblain lupus due to a gain-of-function mutation in STING. Ann. Rheum. Dis. 76, 468–472 (2017).
Keskitalo, S. et al. Novel TMEM173 mutation and the role of disease modifying alleles. Front. Immunol. 10, 2770 (2019).
Li, J., An, S. & Du, Z. Familial interstitial lung disease caused by mutation of the STING1 gene. Front. Pediatr. 8, 543 (2020).
Lin, B. et al. A novel STING1 variant causes a recessive form of STING-associated vasculopathy with onset in infancy (SAVI). J. Allergy Clin. Immunol. 146, 1204–1208 e1206 (2020).
Wan, R. et al. Phenotypic spectrum in recessive STING-associated vasculopathy with onset in infancy: four novel cases and analysis of previously reported cases. Front. Immunol. 13, 1029423 (2022).
Alghamdi, M. A. et al. A novel biallelic STING1 gene variant causing SAVI in two siblings. Front. Immunol. 11, 599564 (2020).
Watkin, L. B. et al. COPA mutations impair ER-Golgi transport and cause hereditary autoimmune-mediated lung disease and arthritis. Nat. Genet. 47, 654–660 (2015).
Lepelley, A. et al. Mutations in COPA lead to abnormal trafficking of STING to the Golgi and interferon signaling. J. Exp. Med. 217, e20200600 (2020).
Delafontaine, S. et al. Heterozygous mutations in the C-terminal domain of COPA underlie a complex autoin fl ammatory syndrome. J. Clin. Invest. 134, e163604 (2024).
Deng, Z. et al. A defect in COPI-mediated transport of STING causes immune dysregulation in COPA syndrome. J. Exp. Med. 217, e20201045 (2020).
Tang, X. et al. STING-associated vasculopathy with onset in infancy in three children with new clinical aspect and unsatisfactory therapeutic responses to tofacitinib. J. Clin. Immunol. 40, 114–122 (2020).
Fremond, M. L. et al. Overview of STING-associated vasculopathy with onset in infancy (SAVI) among 21 patients. J. Allergy Clin. Immunol. Pract. 9, 803–818 e811 (2021).
Melki, I. et al. Disease-associated mutations identify a novel region in human STING necessary for the control of type I interferon signaling. J. Allergy Clin. Immunol. 140, 543–552 e545 (2017).
Ma, M., Mazumder, S., Kwak, H., Adams, M. & Gregory, M. Case report: acute thrombotic microangiopathy in a patient with sting-associated vasculopathy with onset in infancy (SAVI). J. Clin. Immunol. 40, 1111–1115 (2020).
Abid, Q. et al. APOL1-associated collapsing focal segmental glomerulosclerosis in a patient with stimulator of interferon genes (STING)-associated vasculopathy with onset in infancy (SAVI). Am. J. Kidney Dis. 75, 287–290 (2020).
Gurnari, C. et al. Allogeneic hematopoietic cell transplantation for VEXAS syndrome: results of a multicenter study of the EBMT. Blood Adv. 8, 1444–1448 (2024).
Simchoni, N., Vogel, T. P. & Shum, A. K. COPA syndrome from diagnosis to treatment: a clinician’s guide. Rheum. Dis. Clin. North Am. 49, 789–804 (2023).
Zheng, Y. et al. COPA syndrome caused by a novel p.Arg227Cys COPA gene variant. Mol. Genet. Genom. Med. 12, e2309 (2024).
Yamazaki-Nakashimada, M. A. et al. Systemic autoimmunity in a patient with CANDLE syndrome. J. Investig. Allergol. Clin. Immunol. 29, 75–76 (2019).
Goldbach-Mansky, R., Alehashemi, S. & de Jesus, A. A. Emerging concepts and treatments in autoinflammatory interferonopathies and monogenic systemic lupus erythematosus. Nat. Rev. Rheumatol. 21, 22–45 (2025).
David, C. & Fremond, M. L. Lung inflammation in STING-associated vasculopathy with onset in infancy (SAVI). Cells 11, 318 (2022).
Lodi, L. et al. Type I interferon-related kidney disorders. Kidney Int. 101, 1142–1159 (2022).
Sanchez, G. A. M. et al. JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies. J. Clin. Invest. 128, 3041–3052 (2018).
Mansilla-Polo, M. et al. Successful treatment of stimulator of interferon genes-associated vasculopathy of infantile onset SAVI syndrome with anifrolumab. JAMA Dermatol. 160, 899–901 (2024).
Kretzschmar, G. et al. Normalized interferon signatures and clinical improvements by IFNAR1 blocking antibody (Anifrolumab) in patients with type I interferonopathies. J. Clin. Immunol. 45, 31 (2024).
Cetin Gedik, K. et al. The 2021 European Alliance of Associations for Rheumatology/American college of rheumatology points to consider for diagnosis and management of autoinflammatory type I interferonopathies: CANDLE/PRAAS, SAVI, and AGS. Arthritis Rheumatol. 74, 735–751 (2022).
Matucci-Cerinic, C. et al. Baricitinib treatment in children with COPA syndrome. J. Allergy Clin. Immunol. Pract. 12, 2201–2204 (2024).
Martinez, C. et al. HSCT corrects primary immunodeficiency and immune dysregulation in patients with POMP-related autoinflammatory disease. Blood 138, 1896–1901 (2021).
Verhoeven, D. et al. Hematopoietic stem cell transplantation in a patient with proteasome-associated autoinflammatory syndrome (PRAAS). J. Allergy Clin. Immunol. 149, 1120–1127 e1128 (2022).
Louvrier, C. et al. De novo gain-of-function variations in LYN associated with an early-onset systemic autoinflammatory disorder. Arthritis Rheumatol. 75, 468–474 (2023).
Kanderova, V. et al. Early-onset pulmonary and cutaneous vasculitis driven by constitutively active SRC-family kinase HCK. J. Allergy Clin. Immunol. 149, 1464–1472 e1463 (2022).
Ingley, E. Src family kinases: regulation of their activities, levels and identification of new pathways. Biochim. Biophys. Acta 1784, 56–65 (2008).
Keating, G. M. Dasatinib: a review in chronic myeloid leukaemia and Ph+ acute lymphoblastic leukaemia. Drugs 77, 85–96 (2017).
Masters, S. L., Simon, A., Aksentijevich, I. & Kastner, D. L. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease. Annu. Rev. Immunol. 27, 621–668 (2009).
Barzilai, A. et al. Erysipelas-like erythema of familial Mediterranean fever: clinicopathologic correlation. J. Am. Acad. Dermatol. 42, 791–795 (2000).
Dogan, C. S. et al. Prevalence and significance of the MEFV gene mutations in childhood Henoch-Schonlein purpura without FMF symptoms. Rheumatol. Int. 33, 377–380 (2013).
Abbara, S. et al. Vasculitis and familial Mediterranean fever: description of 22 French adults from the juvenile inflammatory rheumatism cohort. Front. Med. 9, 1000167 (2022).
Ben-Chetrit, E. & Yazici, H. Non-thrombocytopenic purpura in familial Mediterranean fever-comorbidity with Henoch–Schonlein purpura or an additional rare manifestation of familial Mediterranean fever? Rheumatology 55, 1153–1158 (2016).
Ozen, S. et al. Polyarteritis nodosa in patients with familial Mediterranean fever (FMF): a concomitant disease or a feature of FMF? Semin. Arthritis Rheum. 30, 281–287 (2001).
Omoyinmi, E., Rowczenio, D., Sebire, N., Brogan, P. A. & Eleftheriou, D. Vasculitis in a patient with mevalonate kinase deficiency (MKD): a case report. Pediatr. Rheumatol. Online J. 19, 161 (2021).
Zhong, L. et al. Phenotype of Takayasu-like vasculitis and cardiopathy in patients with Blau syndrome. Clin. Rheumatol. 43, 1171–1181 (2024).
Khubchandani, R. P. et al. Blau arteritis resembling Takayasu disease with a novel NOD2 mutation. J. Rheumatol. 39, 1888–1892 (2012).
An, J. W. et al. Case report: novel variants in RELA associated with familial Behcet’s-like disease. Front. Immunol. 14, 1127085 (2023).
Moriya, K. et al. Human RELA dominant-negative mutations underlie type I interferonopathy with autoinflammation and autoimmunity. J. Exp. Med. 220, e20212276 (2023).
Papa, R., Penco, F., Volpi, S. & Gattorno, M. Actin remodeling defects leading to autoinflammation and immune dysregulation. Front. Immunol. 11, 604206 (2020).
Vasquez-Echeverri, E. et al. Is your kid actin out? A series of six patients with inherited actin-related protein 2/3 complex subunit 1B deficiency and review of the literature. J. Allergy Clin. Immunol. Pract. 11, 1261–1280 e1268 (2023).
Giardino, S. et al. Hematopoietic stem cell transplantation in ARPC1B deficiency. J. Clin. Immunol. 42, 1535–1544 (2022).
Volpi, S. et al. A combined immunodeficiency with severe infections, inflammation, and allergy caused by ARPC1B deficiency. J. Allergy Clin. Immunol. 143, 2296–2299 (2019).
Taietti, I., Catamero, F., Lodi, L., Giovannini, M. & Castagnoli, R. Inborn errors of immunity with atopic phenotypes in the allergy and immunology clinic: a practical review. Curr. Opin. Allergy Clin. Immunol. https://doi.org/10.1097/ACI.0000000000001059 (2025).
Grayson, P. C., Patel, B. A. & Young, N. S. VEXAS syndrome. Blood 137, 3591–3594 (2021).
Grayson, P. C., Beck, D. B., Ferrada, M. A., Nigrovic, P. A. & Kastner, D. L. VEXAS syndrome and disease taxonomy in rheumatology. Arthritis Rheumatol. 74, 1733–1736 (2022).
Georgin-Lavialle, S. et al. Further characterization of clinical and laboratory features in VEXAS syndrome: large-scale analysis of a multicentre case series of 116 French patients. Br. J. Dermatol. 186, 564–574 (2022).
Ferrada, M. A. et al. Somatic mutations in UBA1 define a distinct subset of relapsing polychondritis patients with VEXAS. Arthritis Rheumatol. 73, 1886–1895 (2021).
Obiorah, I. E. et al. Benign and malignant hematologic manifestations in patients with VEXAS syndrome due to somatic mutations in UBA1. Blood Adv. 5, 3203–3215 (2021).
Borie, R. et al. Pleuropulmonary manifestations of vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic (VEXAS) syndrome. Chest 163, 575–585 (2023).
Collins, J. C. et al. Shared and distinct mechanisms of UBA1 inactivation across different diseases. EMBO J. 43, 1919–1946 (2024).
Ferrada, M. A. et al. Translation of cytoplasmic UBA1 contributes to VEXAS syndrome pathogenesis. Blood 140, 1496–1506 (2022).
Wu, Z. et al. Early activation of inflammatory pathways in UBA1-mutated hematopoietic stem and progenitor cells in VEXAS. Cell Rep. Med. 4, 101160 (2023).
Beck, D. B. et al. Estimated prevalence and clinical manifestations of UBA1 variants associated with VEXAS syndrome in a clinical population. JAMA 329, 318–324 (2023).
Gutierrez-Rodrigues, F. et al. Clonal haematopoiesis across the age spectrum of vasculitis patients with Takayasu’s arteritis, ANCA-associated vasculitis and giant cell arteritis. Ann. Rheum. Dis. 83, 508–517 (2024).
Robinette, M. L. et al. Association of somatic TET2 mutations with giant cell arteritis. Arthritis Rheumatol. 76, 438–443 (2024).
Gutierrez-Rodrigues, F. et al. Spectrum of clonal hematopoiesis in VEXAS syndrome. Blood 142, 244–259 (2023).
Koster, M. J. et al. VEXAS syndrome: clinical, hematologic features and a practical approach to diagnosis and management. Am. J. Hematol. 99, 284–299 (2024).
Hadjadj, J. et al. Efficacy and safety of targeted therapies in VEXAS syndrome: retrospective study from the FRENVEX. Ann. Rheum. Dis. 83, 1358–1367 (2024).
Zavialov, A. V., Yu, X., Spillmann, D., Lauvau, G. & Zavialov, A. V. Structural basis for the growth factor activity of human adenosine deaminase ADA2. J. Biol. Chem. 285, 12367–12377 (2010).
Wouters, M. et al. Human ADA2 deficiency: ten years later. Curr. Allergy Asthma Rep. 24, 477–484 (2024).
Chen, L. et al. Comparison of disease phenotypes and mechanistic insight on causal variants in patients with DADA2. J. Allergy Clin. Immunol. 152, 771–782 (2023).
Deuitch, N. T. et al. TNF inhibition in vasculitis management in adenosine deaminase 2 deficiency (DADA2). J. Allergy Clin. Immunol. 149, 1812–1816 e1816 (2022).
Lee, P. Y. et al. Adenosine deaminase 2 as a biomarker of macrophage activation syndrome in systemic juvenile idiopathic arthritis. Ann. Rheum. Dis. 79, 225–231 (2020).
Schnappauf, O. et al. Sequence-based screening of patients with idiopathic polyarteritis nodosa, granulomatosis with polyangiitis, and microscopic polyangiitis for deleterious genetic variants in ADA2. Arthritis Rheumatol. 73, 512–519 (2021).
Beck, D. B., Werner, A., Kastner, D. L. & Aksentijevich, I. Disorders of ubiquitylation: unchained inflammation. Nat. Rev. Rheumatol. 18, 435–447 (2022).
Shembade, N., Ma, A. & Harhaj, E. W. Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes. Science 327, 1135–1139 (2010).
Vande Walle, L. et al. Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis. Nature 512, 69–73 (2014).
Adrianto, I. et al. Association of a functional variant downstream of TNFAIP3 with systemic lupus erythematosus. Nat. Genet. 43, 253–258 (2011).
Catrysse, L., Vereecke, L., Beyaert, R. & van Loo, G. A20 in inflammation and autoimmunity. Trends Immunol. 35, 22–31 (2014).
Kosmider, O. et al. VEXAS syndrome is characterized by inflammasome activation and monocyte dysregulation. Nat. Commun. 15, 910 (2024).
Balka, K. R. et al. TBK1 and IKKε act redundantly to mediate STING-induced NF-κB responses in myeloid cells. Cell Rep. 31, 107492 (2020).
Siedel, H., Roers, A., Rosen-Wolff, A. & Luksch, H. Type I interferon-independent T cell impairment in a Tmem173 N153S/WT mouse model of STING associated vasculopathy with onset in infancy (SAVI). Clin. Immunol. 216, 108466 (2020).
Deng, Z. et al. A defect in thymic tolerance causes T cell-mediated autoimmunity in a murine model of COPA syndrome. J. Immunol. 204, 2360–2373 (2020).
Gao, K. M. et al. Endothelial cell expression of a STING gain-of-function mutation initiates pulmonary lymphocytic infiltration. Cell Rep. 43, 114114 (2024).
Aksentijevich, I. & Schnappauf, O. Molecular mechanisms of phenotypic variability in monogenic autoinflammatory diseases. Nat. Rev. Rheumatol. 17, 405–425 (2021).
Muratore, F. et al. VEXAS syndrome: a case series from a single-center cohort of italian patients with vasculitis. Arthritis Rheumatol. 74, 665–670 (2022).
Hashem, H. et al. Hematopoietic cell transplantation cures adenosine deaminase 2 deficiency: report on 30 patients. J. Clin. Immunol. 41, 1633–1647 (2021).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks Pui Lee, Raju Khubchandani and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gül, A., Aksentijevich, I., Brogan, P. et al. The pathogenesis, clinical presentations and treatment of monogenic systemic vasculitis. Nat Rev Rheumatol 21, 414–425 (2025). https://doi.org/10.1038/s41584-025-01250-9
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41584-025-01250-9
This article is cited by
-
Clinical features and genetic analysis of A20 haploinsufficiency
Orphanet Journal of Rare Diseases (2025)
-
Reply to ‘Inborn errors of immunity and AAV: a complex picture’
Nature Reviews Rheumatology (2025)
-
Challenges in the diagnosis, classification and prognosis of ANCA-associated vasculitis
Nature Reviews Rheumatology (2025)
-
Inborn errors of immunity and AAV: a complex picture
Nature Reviews Rheumatology (2025)
-
Polyarteritis nodosa presenting with pancreatic-artery rupture and co-existing MEFV and ADA2 mutation: clinicopathological and genomic insights from a case report
Virchows Archiv (2025)