Abstract
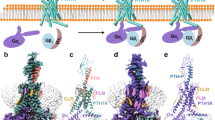
Parathyroid hormone (PTH) regulates bone homeostasis. Intermittent exposure to PTH results in bone formation being greater than bone resorption, and this effect has been harnessed through the development of agonists of the PTH and PTH-related protein type 1 receptor (PTH1R) to treat osteoporosis. Teriparatide, an analogue of the first 34 amino acids of PTH, and abaloparatide, which resembles PTH-related protein (PTHrP) in structure, are PTH1R agonists currently in clinical use. Both medications have been shown to increase bone mineral density at the lumbar spine, femoral neck and total hip. Randomized controlled trials with teriparatide or abaloparatide have also provided evidence of reduction in vertebral and non-vertebral fractures. The ACTIVE trial suggested slightly greater efficacy for major osteoporotic fractures (as an exploratory end point) for abaloparatide than for teriparatide. A similar potential superiority was suggested for hip fracture in a real-world, observational study. Side effects of these medications are usually transient, and although a risk of osteosarcoma was suggested by studies using murine models, no such risk has been observed in extensive human studies. Overall, both teriparatide and abaloparatide have demonstrated convincing clinical effectiveness and cost-effectiveness, with a reassuring safety profile. Potential differences in their effects on bone mineral density and their antifracture effects offer avenues for differentiation but require further validation in appropriately designed studies.
Key points
-
Parathyroid hormone type 1 receptor (PTH1R) agonists stimulate bone formation and effectively reduce the risk of vertebral and non-vertebral fractures.
-
The PTH1R agonists teriparatide and abaloparatide act via intermittent PTH1R stimulation, as opposed to the constant PTH1R stimulation seen in hyperparathyroidism.
-
The safety profiles of teriparatide and abaloparatide are favourable, with previous concerns regarding osteosarcoma in murine models not born out in humans, and with cardiovascular safety having been consistently demonstrated.
-
Exploratory analysis of data from the ACTIVE trial suggests that abaloparatide might have greater efficacy in reducing the risk of major osteoporotic fractures than teriparatide.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
28 August 2025
In the version of the article initially published, footnotes for equally contributing authors (now listing Nicholas Fuggle and René Rizzoli) and jointly supervising authors (now listing Jean-Yves Reginster and Nicholas C. Harvey) were incorrect and are now amended in the HTML and PDF versions of the article.
30 September 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41584-025-01314-w
References
Drake, M. T., Clarke, B. L. & Khosla, S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin. Proc. 83, 1032–1045 (2008).
McClung, M. R. et al. Denosumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 354, 821–831 (2006).
Riggs, B. L. & Hartmann, L. C. Selective estrogen-receptor modulators — mechanisms of action and application to clinical practice. N. Engl. J. Med. 348, 618–629 (2003).
Rozenberg, S. et al. Is there a role for menopausal hormone therapy in the management of postmenopausal osteoporosis? Osteoporos. Int. 31, 2271–2286 (2020).
Marino, S. & Bellido, T. PTH receptor signalling, osteocytes and bone disease induced by diabetes mellitus. Nat. Rev. Endocrinol. 20, 661–672 (2024).
Miller, P. D. et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA 316, 722–733 (2016).
Neer, R. M. et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 344, 1434–1441 (2001).
Tabatabai, L. et al. Comparative effectiveness of abaloparatide and teriparatide in women 50 years of age and older: update of a real-world retrospective analysis. Endocr. Pract. 31, 159–168 (2024).
Veronese, N. et al. Recommendations for the optimal use of bone forming agents in osteoporosis. Aging Clin. Exp. Res. 36, 167 (2024).
Fuggle, N. R. et al. Evidence-based guideline for the management of osteoporosis in men. Nat. Rev. Rheumatol. 20, 241–251 (2024).
Tabacco, G. & Bilezikian, J. P. Osteoanabolic and dual action drugs. Br. J. Clin. Pharmacol. 85, 1084–1094 (2019).
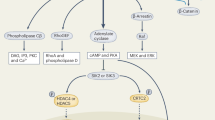
Chiavistelli, S., Giustina, A. & Mazziotti, G. Parathyroid hormone pulsatility: physiological and clinical aspects. Bone Res. 3, 14049 (2015).
Shimonty, A., Bonewald, L. F. & Huot, J. R. Metabolic health and disease: a role of osteokines? Calcif. Tissue Int. 113, 21–38 (2023).
Gardella, T. J. & Vilardaga, J. P. International Union of Basic and Clinical Pharmacology. XCIII. The parathyroid hormone receptors — family B G protein-coupled receptors. Pharmacol. Rev. 67, 310–337 (2015).
Gelbert, L. et al. Chromosomal localization of the parathyroid hormone/parathyroid hormone-related protein receptor gene to human chromosome 3p21.1-p24.2. J. Clin. Endocrinol. Metab. 79, 1046–1048 (1994).
Burtis, W. J. Parathyroid hormone-related protein: structure, function, and measurement. Clin. Chem. 38, 2171–2183 (1992).
Khosla, S. & Hofbauer, L. C. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 5, 898–907 (2017).
Ukon, Y. et al. Molecular-based treatment strategies for osteoporosis: a literature review. Int. J. Mol. Sci. 20, 2557 (2019).
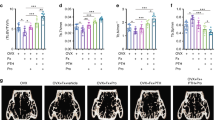
Ma, Y. L. et al. Teriparatide increases bone formation in modeling and remodeling osteons and enhances IGF‐II immunoreactivity in postmenopausal women with osteoporosis. J. Bone Miner. Res. 21, 855–864 (2009).
Dempster, D. W. et al. On the mechanism of cancellous bone preservation in postmenopausal women with mild primary hyperparathyroidism. J. Clin. Endocrinol. Metab. 84, 1562–1566 (1999).
Rosen, C. J. & Bilezikian, J. P. Anabolic therapy for osteoporosis. J. Clin. Endocrinol. Metab. 86, 957–964 (2001).
Dempster, D. W. et al. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J. Bone Miner. Res. 16, 1846–1853 (2001).
Dempster, D. W. et al. Remodeling- and modeling-based bone formation with teriparatide versus denosumab: a longitudinal analysis from baseline to 3 months in the AVA study. J. Bone Miner. Res. 33, 298–306 (2018).
Miller, P. D. et al. Abaloparatide: an anabolic treatment to reduce fracture risk in postmenopausal women with osteoporosis. Curr. Med. Res. Opin. 36, 1861–1872 (2020).
Hattersley, G. et al. Binding selectivity of abaloparatide for PTH-type-1-receptor conformations and effects on downstream signaling. Endocrinology 157, 141–149 (2016).
Bhattacharyya, S., Pal, S. & Chattopadhyay, N. Abaloparatide, the second generation osteoanabolic drug: molecular mechanisms underlying its advantages over the first-in-class teriparatide. Biochem. Pharmacol. 166, 185–191 (2019).
European Medicines Agency. Summary of product characteristics: Forsteo. https://www.ema.europa.eu/en/medicines/human/EPAR/forsteo (2013).
European Medicines Agency. Summary of product characteristics: Eladynos. https://www.ema.europa.eu/en/medicines/human/EPAR/eladynos (2022).
Boonen, S. et al. Safety and efficacy of teriparatide in elderly women with established osteoporosis: bone anabolic therapy from a geriatric perspective. J. Am. Geriatr. Soc. 54, 782–789 (2006).
Harvey, N. C. et al. FRAX and the effect of teriparatide on vertebral and non-vertebral fracture. Osteoporos. Int. 26, 2677–2684 (2015).
Prince, R. et al. Sustained nonvertebral fragility fracture risk reduction after discontinuation of teriparatide treatment. J. Bone Miner. Res. 20, 1507–1513 (2005).
Kendler, D. L. et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 391, 230–240 (2018).
Díez-Pérez, A. et al. Effects of teriparatide on hip and upper limb fractures in patients with osteoporosis: a systematic review and meta-analysis. Bone 120, 1–8 (2019).
Gasser, J. A. et al. PTH and interactions with bisphosphonates. J. Musculoskelet. Neuronal Interact. 1, 53–56 (2000).
Black, D. M. et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N. Engl. J. Med. 349, 1207–1215 (2003).
Cosman, F. et al. Effects of intravenous zoledronic acid plus subcutaneous teriparatide [rhPTH(1-34)] in postmenopausal osteoporosis. J. Bone Miner. Res. 26, 503–511 (2011).
Tsai, J. N. et al. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet 382, 50–56 (2013).
Cosman, F. et al. Effects of teriparatide in postmenopausal women with osteoporosis on prior alendronate or raloxifene: differences between stopping and continuing the antiresorptive agent. J. Clin. Endocrinol. Metab. 94, 3772–3780 (2009).
Leder, B. Z. et al. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet 386, 1147–1155 (2015).
Tsai, J. N. et al. Effects of denosumab and teriparatide transitions on bone microarchitecture and estimated strength: the DATA-Switch HR-pQCT study. J. Bone Miner. Res. 34, 976 (2019).
Cosman, F., Nieves, J. W. & Dempster, D. W. Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J. Bone Miner. Res. 32, 198–202 (2017).
Chandran, M. The why and how of sequential and combination therapy in osteoporosis. A review of the current evidence. Arch. Endocrinol. Metab. 66, 724–738 (2022).
Langdahl, B. L. et al. Reduction in fracture rate and back pain and increased quality of life in postmenopausal women treated with teriparatide: 18-month data from the European Forsteo Observational Study (EFOS). Calcif. Tissue Int. 85, 484–493 (2009).
Fahrleitner-Pammer, A. et al. Fracture rate and back pain during and after discontinuation of teriparatide: 36-month data from the European Forsteo Observational Study (EFOS). Osteoporos. Int. 22, 2709–2719 (2011).
Wang, Y. K. et al. Effects of teriparatide versus alendronate for treatment of postmenopausal osteoporosis: a meta-analysis of randomized controlled trials. Medicine 96, e6970 (2017).
McCloskey, E. V. et al. The effect of abaloparatide-SC on fracture risk is independent of baseline FRAX fracture probability: a post hoc analysis of the ACTIVE study. J. Bone Miner. Res. 32, 1625–1631 (2017).
Winzenrieth, R. et al. Differential effects of abaloparatide and teriparatide on hip cortical volumetric BMD by DXA-based 3D modeling. Osteoporos. Int. 32, 575–583 (2021).
Bone, H. G. et al. ACTIVExtend: 24 months of alendronate after 18 months of abaloparatide or placebo for postmenopausal osteoporosis. J. Clin. Endocrinol. Metab. 103, 2949–2957 (2018).
Greenspan, S. L. et al. Abaloparatide followed by alendronate in women ≥80 years with osteoporosis: post hoc analysis of ACTIVExtend. Menopause 27, 1137–1142 (2020).
Lewiecki, E. M. et al. Efficacy and safety of transdermal abaloparatide in postmenopausal women with osteoporosis: a randomized study. J. Bone Miner. Res. 38, 1404–1414 (2023).
Leder, B. Z. et al. Effects of abaloparatide, a human parathyroid hormone-related peptide analog, on bone mineral density in postmenopausal women with osteoporosis. J. Clin. Endocrinol. Metab. 100, 697–706 (2015).
Hong, P. et al. Is abaloparatide more efficacious on increasing bone mineral density than teriparatide for women with postmenopausal osteoporosis? An updated meta-analysis. J. Orthop. Surg. Res. 18, 116 (2023).
Beaudart, C. et al. PTH1 receptor agonists for fracture risk: a systematic review and network meta-analysis. Osteoporos. Int. 36, 951–967 (2025).
Orwoll, E. S. et al. The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J. Bone Miner. Res. 18, 9–17 (2003).
Kurland, E. S. et al. Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J. Clin. Endocrinol. Metab. 85, 3069–3076 (2000).
Beaudart, C. et al. Efficacy of osteoporosis pharmacological treatments in men: a systematic review and meta-analysis. Aging Clin. Exp. Res. 35, 1789–1806 (2023).
Kaufman, J. M. et al. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos. Int. 16, 510–516 (2005).
Finkelstein, J. S. et al. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N. Engl. J. Med. 349, 1216–1226 (2003).
Czerwinski, E. et al. The efficacy and safety of abaloparatide-SC in men with osteoporosis: a randomized clinical trial. J. Bone Miner. Res. 37, 2435–2442 (2022).
Towler, D. A. Parathyroid hormone-PTH1R signaling in cardiovascular disease and homeostasis. Trends Endocrinol. Metab. 35, 648–660 (2024).
Lindquist, M. VigiBase, the WHO global ICSR database system: basic facts. Drug. Inf. J. 42, 409–419 (2008).
Rodríguez, A. J., Nerlekar, N. & Ebeling, P. R. Cardiac adverse events in bisphosphonate and teriparatide users: an international pharmacovigilance study. Bone 168, 116647 (2023).
Jolette, J. et al. Comparing the incidence of bone tumors in rats chronically exposed to the selective PTH type 1 receptor agonist abaloparatide or PTH(1-34). Regul. Toxicol. Pharmacol. 86, 356–365 (2017).
Kuijpers, G. et al. Recombinant human parathyroid hormone. Preclinical data on rat osteosarcoma were not dismissed. BMJ 324, 1218 (2002). Author reply 1218.
Gilsenan, A. et al. Teriparatide did not increase adult osteosarcoma incidence in a 15-year US postmarketing surveillance study. J. Bone Miner. Res. 36, 244–251 (2021).
Abdulelah, A. A. et al. The risk of developing osteosarcoma after teriparatide use: a systematic review. Orthop. Res. Rev. 15, 191–198 (2023).
McDonald, C. L. et al. Treatment of osteoporosis with anabolic agents and the risk of primary bone cancers: a study of 44,728 patients treated with teriparatide and abaloparatide. J. Am. Acad. Orthop. Surg. 31, 520–528 (2023).
Cosman, F. et al. Cardiovascular safety of abaloparatide in postmenopausal women with osteoporosis: analysis from the ACTIVE phase 3 trial. J. Clin. Endocrinol. Metab. 105, 3384–3395 (2020).
Cosman, F. et al. Comparative effectiveness and cardiovascular safety of abaloparatide and teriparatide in postmenopausal women new to anabolic therapy: a US Administrative Claims Database study. Osteoporos. Int. 33, 1703–1714 (2022).
Seeto, A. H. et al. Evidence for the cardiovascular effects of osteoporosis treatments in randomized trials of post-menopausal women: a systematic review and Bayesian network meta-analysis. Bone 167, 116610 (2023).
Taheri, S. et al. Teriparatide in the treatment of severe postmenopausal osteoporosis: a cost-utility analysis. Iran. J. Pharm. Res. 18, 1073–1085 (2019).
Borgström, F. et al. The societal burden of osteoporosis in Sweden. Bone 40, 1602–1609 (2007).
Murphy, D. R. et al. The cost effectiveness of teriparatide as a first-line treatment for glucocorticoid-induced and postmenopausal osteoporosis patients in Sweden. BMC Musculoskelet. Disord. 13, 213 (2012).
Ebadi Fard Azar, A. A. et al. Cost-effectiveness of teriparatide compared with alendronate and risedronate for the treatment of postmenopausal osteoporosis patients in Iran. Med. J. Islam. Repub. Iran. 31, 39 (2017).
Silverman, S. et al. Denosumab for elderly men with osteoporosis: a cost-effectiveness analysis from the US payer perspective. J. Osteoporos. 2015, 627631 (2015).
Mori, T. et al. Cost-effectiveness of sequential daily teriparatide/weekly alendronate compared with alendronate monotherapy for older osteoporotic women with prior vertebral fracture in Japan. Arch. Osteoporos. 16, 72 (2021).
Mori, T., Crandall, C. J. & Ganz, D. A. Cost-effectiveness of sequential teriparatide/alendronate versus alendronate-alone strategies in high-risk osteoporotic women in the US: analyzing the impact of generic/biosimilar teriparatide. JBMR 3, e10233 (2019).
Hiligsmann, M. et al. Cost-effectiveness of sequential treatment with abaloparatide followed by alendronate vs. alendronate monotherapy in women at increased risk of fracture: a US payer perspective. Semin. Arthritis Rheum. 50, 394–400 (2020).
Yu, G. et al. A systematic review of cost-effectiveness analyses of sequential treatment for osteoporosis. Osteoporos. Int. 34, 641–658 (2023).
Le, Q. A. et al. Cost-effectiveness analysis of sequential treatment of abaloparatide followed by alendronate versus teriparatide followed by alendronate in postmenopausal women with osteoporosis in the United States. Ann. Pharmacother. 53, 134–143 (2019).
Hiligsmann, M. et al. Cost-effectiveness of sequential treatment with abaloparatide vs. teriparatide for United States women at increased risk of fracture. Semin. Arthritis Rheum. 49, 184–196 (2019).
Hiligsmann, M. et al. Comparison of the cost-effectiveness of sequential treatment with abaloparatide in US men and women at very high risk of fractures. Aging Clin. Exp. Res. 36, 14 (2024).
Borgström, F. et al. Cost-effectiveness intervention thresholds for romosozumab and teriparatide in the treatment of osteoporosis in the UK. Osteoporos. Int. 35, 2183–2193 (2024).
Cosman, F. et al. Goal-directed osteoporosis treatment: ASBMR/BHOF task force position statement 2024. J. Bone Miner. Res. 39, 1393–1405 (2024).
Curtis, E. M. et al. Management of patients at very high risk of osteoporotic fractures through sequential treatments. Aging Clin. Exp. Res. 34, 695–714 (2022).
Kanis, J. A. et al. Algorithm for the management of patients at low, high and very high risk of osteoporotic fractures. Osteoporos. Int. 31, 1–12 (2020).
Händel, M. N. et al. Fracture risk reduction and safety by osteoporosis treatment compared with placebo or active comparator in postmenopausal women: systematic review, network meta-analysis, and meta-regression analysis of randomised clinical trials. BMJ 381, e068033 (2023).
Kellier-Steele, N. et al. Assessing the incidence of osteosarcoma among teriparatide-treated patients using linkage of commercial pharmacy and state cancer registry data, contributing to the removal of boxed warning and other labeling changes. Bone 160, 116394 (2022).
Acknowledgements
This ESCEO Working Group was funded by the ESCEO. The ESCEO receives unrestricted educational grants to support its educational and scientific activities from non-governmental organisations, not-for-profit organisations, non-commercial or corporate partners. The choice of topics, participants, content and agenda of the Working Groups as well as the writing, editing, submission and reviewing of the manuscript are the sole responsibility of the ESCEO, without any influence from third parties. This work was supported by the Distinguished Scientist Fellowship Program (DSFP) of the King Saud University, Riyadh, Kingdom of Saudi Arabia.
Author information
Authors and Affiliations
Contributions
N.F., N.C.H., J.Y.-R. and R.R. wrote the initial draft of the article. All authors contributed substantially to discussion of the content, and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
N.F. has received honoraria and speaker fees from UCB and Viatris, and travel bursaries from Eli Lilly and Pfizer. B.C. has received occasional fees as an expert or speaker from Alexion, Amgen, Aptissen, Expanscience, Lilly, Kyowa Kirin, Novartis, Theramex, UCB and Viatris. E.M.C. has received speaker fees from Eli Lilly, Thornton and Ross, and UCB, and travel bursaries or conference support from Amgen and Eli Lilly. M.H. has received research grants (paid to his institution) from Angelini Pharma and Radius Health, and lecture fees from IBSA (paid to his institution) and Mylan Pharmaceuticals, and was grant adviser for Pfizer (paid to his institution). N.V. declares personal fees from Bayer, Fidia, Nestlè and Viatris. B.H.A. reports research grants from Amgen and UCB, and honoraria from Amgen, Theramex and UCB. M.L.B. declares honoraria from Amgen, Ascendis, Bruno Farmaceutici, Calcilytix and Kyowa Kirin, grants or speaker fees from Alexion, Amgen, Amolyt, Bruno Farmaceutici, CoGeDi, Echolight, Gedeon Richter, Kyowa Kirin, Monte Rosa Therapeutics and UCB, and consultancy for Aboca, Alexion, Amolyt, Bruno Farmaceutici, Calcilytix, Echolight, Enterabio, Kyowa Kirin, Personal Genomics and Septern. O.B. has received consulting or lecture fees from Amgen, Aptissen, Biophytis, IBSA, Mylan, Novartis, Nutricia, Orifarm, Sanofi, UCB and Viatris outside the submitted work. E. Casado has received honoraria and speaker fees from FAES, Gedeon-Richter, STADA, Theramex and UCB, and travel bursaries from Amgen, Rubió, STADA and Theramex. M.C. has received honoraria and travel grants from Amgen and Promedius AI solutions. P.D'A. declares research grants and honoraria from ErreKappa, Nestlé, OM Pharma and Schwabe Pharma. P.R.E. declares research grants from Amgen, Alexion and Sanofi, and honoraria from Amgen, Alexion and Kyowa Kirin. J.A.K. is a director of Osteoporosis Research, which maintains FRAX. A.K. declares honoraria for scientific advisory board, research grants and speakers fees from AgNovos bioscience, Alexion, Amgen, Celltrion Deutschland, Echolight, Eli Lilly, Ipsen, Imaging Biopsy Lab (IBL), Kyowa Kirin, Merit Medical, new4med, Novartis, NovoNordisk, Roche, Sandoz, Servier, Stadapharm, Theramex and UCB. E.McC. is a director of Osteoporosis Research, which maintains FRAX, and has also received honoraria and research funding from Amgen, Fresenius Kabi, Lilly, ObsEva, Radius Pharma, Theramex and UCB. M.McC. declares consulting fees or honoraria from Amgen, Alexion, Pfizer and UCB. O.M. declares lecture fees or honoraria from Bottu Johnson and Johnson, Pfizer and Sanofi. J.-Y.R. declares speaker’s Bureau for Radius Health and Theramex, and consultancy agreement for Theramex. N.C.H. has received personal fees, consultancy, lecture fees or honoraria from Alliance for Better Bone Health, AMGEN, MSD, Eli Lilly, Internis Pharma, Kyowa Kirin, Servier, Shire, Consilient Healthcare, Theramex and UCB outside the submitted work. R.R., C.B., J.-M.K, N.A.-D., M.A., N.B., C. Campusano, E. Cavalier, C. Cooper, B.D.-H., R.M., N.N., R.P. R., F.R., S.S. S.T., and L.Z. have no competing interests to declare.
Peer review
Peer review information
Nature Reviews Rheumatology thanks Torben Harsløf and Bart Clarke for their contribution to the peer review of this work.
Additional information
Informed consent
This narrative article contains no original data and thus issues of ethics, informed consent and patient confidentiality do not apply.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fuggle, N., Rizzoli, R., Beaudart, C. et al. Parathyroid hormone receptor agonists in the management of osteoporosis. Nat Rev Rheumatol 21, 599–611 (2025). https://doi.org/10.1038/s41584-025-01287-w
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41584-025-01287-w
This article is cited by
-
PBX1 promotes osteoporosis by upregulating HMGB1 to suppress osteogenic differentiation of bone marrow mesenchymal stem cells
Stem Cell Research & Therapy (2025)