Abstract
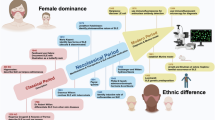
Childhood-onset systemic lupus erythematosus (SLE) is associated with more active disease trajectories, increased cardiovascular risk, earlier development of organ damage (which commonly affects the kidney, central nervous and musculoskeletal systems) and increased use of glucocorticoids and immunosuppressive treatments than adult-onset SLE. However, the understanding of immunopathogenic mechanisms in childhood-onset SLE is far less established than in adult-onset disease. Technological advances over the past decade have accelerated progress in understanding the immune, genetic, epigenetic, metabolic and proteomic profiles of childhood-onset SLE, and have also established the mechanistic roles of immune dysregulation, interferon signalling, biological sex, gender and ethnicity in shaping disease heterogeneity. These insights have led to the elucidation of the mechanisms that drive the increased severity of childhood-onset SLE and point towards new pathways for personalized therapeutic approaches aimed at improving long-term outcomes and quality of life for patients.
Key points
-
Childhood-onset systemic lupus erythematosus (SLE) is more severe than adult-onset SLE, with earlier disease onset, more organ involvement, and higher morbidity and mortality.
-
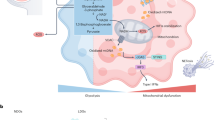
Genetic and epigenetic studies in childhood-onset SLE have revealed age-enriched mechanisms, including monogenic and polygenic contributions, with heightened type I interferon signalling and impaired apoptotic body clearance having central roles.
-
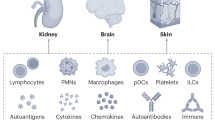
Immune profiling technologies have enabled unique immune cell signatures and organ-specific inflammation patterns to be uncovered in childhood-onset SLE, advancing understanding of disease mechanisms and heterogeneity to guide future precision medicine strategies.
-
Novel biomarkers, notably urinary, lipid and neuroinflammatory markers, have shown promise for early detection and monitoring of lupus nephritis, cardiovascular disease and central nervous system involvement in childhood-onset SLE.
-
Treatments for childhood-onset SLE increasingly include biologic drugs and targeted therapies, although access remains limited; ongoing trials and approval of paediatric-specific treatments and guidelines are essential for therapeutic equity.
-
Future research must prioritize inclusive, multi-ethnic studies, age-specific diagnostic consideration and expanded paediatric clinical trial infrastructure to enable personalized, equitable care for children with childhood-onset SLE.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hiraki, L. T. et al. Prevalence, incidence, and demographics of systemic lupus erythematosus and lupus nephritis from 2000 to 2004 among children in the US Medicaid beneficiary population. Arthritis Rheum. 64, 2669–2676 (2012).
Barber, M. R. W. et al. Global epidemiology of systemic lupus erythematosus. Nat. Rev. Rheumatol. 17, 515–532 (2021).
Valenzuela-Almada, M. O. et al. Epidemiology of childhood-onset systemic lupus erythematosus: a population-based study. Arthritis Care Res. 74, 728–732 (2022).
Ambrose, N. et al. Differences in disease phenotype and severity in SLE across age groups. Lupus 25, 1542–1550 (2016).
Tucker, L. B. et al. Adolescent onset of lupus results in more aggressive disease and worse outcomes: results of a nested matched case-control study within LUMINA, a multiethnic US cohort (LUMINA LVII). Lupus 17, 314–322 (2008).
Hersh, A. O. et al. Differences in long-term disease activity and treatment of adult patients with childhood- and adult-onset systemic lupus erythematosus. Arthritis Rheum. 61, 13–20 (2009).
Groot, N. et al. European evidence-based recommendations for diagnosis and treatment of childhood-onset systemic lupus erythematosus: the SHARE initiative. Ann. Rheum. Dis. 76, 1788–1796 (2017).
Lanças, S. H. S. et al. A Brazilian single-centre series comparision of childhood, adult and late onset systemic lupus erythematosus. Lupus 34, 1110–1118 (2025).
Manzi, S. et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am. J. Epidemiol. 145, 408–415 (1997).
Hersh, A. O. et al. Childhood-onset disease as a predictor of mortality in an adult cohort of patients with systemic lupus erythematosus. Arthritis Care Res. 62, 1152–1159 (2010).
Brunner, H. I. et al. Treatment patterns in paediatric and adult patients with SLE: a retrospective claims database study in the USA. Lupus Sci. Med. 10, e000817 (2023).
Aljaberi, N. et al. Performance of the new 2019 European League Against Rheumatism/American College of Rheumatology Classification criteria for systemic lupus erythematosus in children and young adults. Arthritis Care Res. 73, 580–585 (2021).
Brunner, H. I. et al. Disease outcomes and ovarian function of childhood-onset systemic lupus erythematosus. Lupus 15, 198–206 (2006).
Groot, N. et al. Long-term clinical outcomes in a cohort of adults with childhood-onset systemic lupus erythematosus. Arthritis Rheumatol. 71, 290–301 (2019).
Smith, E. M. D. et al. Attainment of low disease activity and remission targets reduces the risk of severe flare and new damage in childhood Lupus. Rheumatology 61, 3378–3389 (2022).
Zen, M. et al. Lupus low disease activity state is associated with a decrease in damage progression in Caucasian patients with SLE, but overlaps with remission. Ann. Rheum. Dis. 77, 104–110 (2018).
Medina-Quiñones, C. V. et al. Analysis of complete remission in systemic lupus erythematosus patients over a 32-year period. Arthritis Care Res. 68, 981–987 (2016).
Golder, V. et al. Frequency and predictors of the lupus low disease activity state in a multi-national and multi-ethnic cohort. Arthritis Res. Ther. 18, 260 (2016).
Choi, M. Y. & Ma, C. Making a big impact with small datasets using machine-learning approaches. Lancet Rheumatol. 2, e451–e452 (2020).
Trindade, V. C. et al. An update on the management of childhood-onset systemic lupus erythematosus. Paediatr. Drugs 23, 331–347 (2021).
Ghodke-Puranik, Y., Olferiev, M. & Crow, M. K. Systemic lupus erythematosus genetics: insights into pathogenesis and implications for therapy. Nat. Rev. Rheumatol. 20, 635–648 (2024).
Taylor, K. E. et al. Risk alleles for systemic lupus erythematosus in a large case-control collection and associations with clinical subphenotypes. PLoS Genet. 7, e1001311 (2011).
Wang, Q. et al. High-throughput identification of functional regulatory SNPs in systemic lupus erythematosus. Nat. Commun. 15, 6804 (2024).
Charras, A. et al. Genetic and epigenetic factors shape phenotypes and outcomes in systemic lupus erythematosus — focus on juvenile-onset systemic lupus erythematosus. Curr. Opin. Rheumatol. 37, 149–163 (2025).
Chen, Y. J. et al. Polygenic risk score predicts earlier-onset adult systemic lupus erythematosus and first-year renal diseases in a Taiwanese cohort. RMD Open 10, e003293 (2024).
Webb, R. et al. Early disease onset is predicted by a higher genetic risk for lupus and is associated with a more severe phenotype in lupus patients. Ann. Rheum. Dis. 70, 151–156 (2011).
Webber, D. et al. Association of systemic lupus erythematosus (SLE) genetic susceptibility loci with lupus nephritis in childhood-onset and adult-onset SLE. Rheumatology 59, 90–98 (2020).
Sinicato, N. A. et al. Familial aggregation of childhood- and adulthood-onset systemic lupus erythematosus. Arthritis Care Res. 72, 1147–1151 (2020).
Renaudineau, Y. et al. Type I interferon associated epistasis may contribute to early disease-onset and high disease activity in juvenile-onset lupus. Clin. Immunol. 262, 110194 (2024).
Renaudineau, Y. et al. Common Toll-like receptor 7 variants define disease risk and phenotypes in juvenile-onset systemic lupus erythematosus. J. Autoimmun. 155, 103451 (2025).
Natoli, V. et al. Genetic variability associates with ancestry, age at disease onset, organ involvement and disease severity in juvenile-onset systemic lupus erythematosus. Clin. Immunol. 279, 110540 (2025).
Carlomagno, R. et al. Genetics of childhood-onset systemic lupus erythematosus. Arthritis Rheumatol. 77, 1524–1532 (2025).
Belot, A. et al. Contribution of rare and predicted pathogenic gene variants to childhood-onset lupus: a large, genetic panel analysis of British and French cohorts. Lancet Rheumatol. 2, e99–e109 (2020).
Belot, A. & Cimaz, R. Monogenic forms of systemic lupus erythematosus: new insights into SLE pathogenesis. Pediatr. Rheumatol. Online J. 10, 21 (2012).
Charras, A. et al. Panel sequencing links rare, likely damaging gene variants with distinct clinical phenotypes and outcomes in juvenile-onset SLE. Rheumatology 62, SI210–SI225 (2023).
Lintner, K. E. et al. Early components of the complement classical activation pathway in human systemic autoimmune diseases. Front. Immunol. 7, 36 (2016).
Brown, G. J. et al. TLR7 gain-of-function genetic variation causes human lupus. Nature 605, 349–356 (2022).
David, C. et al. Gain-of-function human UNC93B1 variants cause systemic lupus erythematosus and chilblain lupus. J. Exp. Med. 221, e20232066 (2024).
Al-Azab, M. et al. Genetic variants in UNC93B1 predispose to childhood-onset systemic lupus erythematosus. Nat. Immunol. 25, 969–980 (2024).
Xie, C. et al. De novo PACSIN1 gene variant found in childhood lupus and a role for PACSIN1/TRAF4 complex in toll-like receptor 7 activation. Arthritis Rheumatol. 75, 1058–1071 (2023).
Sestan, M. et al. The role of genetic risk factors in pathogenesis of childhood-onset systemic lupus erythematosus. Curr. Issues Mol. Biol. 45, 5981–6002 (2023).
Tusseau, M. et al. DNASE1L3 deficiency, new phenotypes, and evidence for a transient type I IFN signaling. J. Clin. Immunol. 42, 1310–1320 (2022).
Sisirak, V. et al. Digestion of chromatin in apoptotic cell microparticles prevents autoimmunity. Cell 166, 88–101 (2016).
Picard, C. et al. Anti-C1q autoantibodies as markers of renal involvement in childhood-onset systemic lupus erythematosus. Pediatr. Nephrol. 32, 1537–1545 (2017).
Hartl, J. et al. Autoantibody-mediated impairment of DNASE1L3 activity in sporadic systemic lupus erythematosus. J. Exp. Med. 218, e20201138 (2021).
Jeanpierre, M. et al. Haploinsufficiency in PTPN2 leads to early-onset systemic autoimmunity from Evans syndrome to lupus. J. Exp. Med. 221, e20232337 (2024).
Hadjadj, J. et al. Early-onset autoimmunity associated with SOCS1 haploinsufficiency. Nat. Commun. 11, 5341 (2020).
Wang, Y. et al. Phenotypic characterization of patients with activated PI3Kδ syndrome 1 presenting with features of systemic lupus erythematosus. Genes. Dis. 8, 907–917 (2021).
Thuner, J., Cognard, J. & Belot, A. How to treat monogenic SLE? Best. Pract. Res. Clin. Rheumatol. 38, 101962 (2024).
Belot, A. et al. Protein kinase cδ deficiency causes Mendelian systemic lupus erythematosus with B cell-defective apoptosis and hyperproliferation. Arthritis Rheum. 65, 2161–2171 (2013).
Misztal, M. C. et al. Genome-wide sequencing identified rare genetic variants for childhood-onset monogenic lupus. J. Rheumatol. 50, 671–675 (2023).
Lewandowski, L. et al. Next generation sequencing analysis reveals complex genetic architecture of childhood-onset systemic lupus erythematosus. Lupus Sci. Med. 12, e001475 (2025).
Belot, A. et al. How (ultra-)rare gene variants improve our understanding of more common autoimmune and inflammatory diseases. ACR Open. Rheumatol. 7, e70003 (2025).
Keshavarz-Fathi, M. et al. DNA methylation of CD70 promoter in juvenile systemic lupus erythematosus. Fetal Pediatr. Pathol. 41, 58–67 (2022).
Yeung, K. S. et al. Cell lineage-specific genome-wide DNA methylation analysis of patients with paediatric-onset systemic lupus erythematosus. Epigenetics 14, 341–351 (2019).
Hanaei, S. et al. The status of FOXP3 gene methylation in pediatric systemic lupus erythematosus. Allergol. Immunopathol. 48, 332–338 (2020).
Keshavarz-Fathi, M. et al. Aberrant DNA methylation of the promoters of JAK2 and SOCS3 in juvenile systemic lupus erythematosus. Eur. Cytokine Netw. 32, 48–54 (2021).
Wang, J. et al. DNA methylation of IFI44L as a potential blood biomarker for childhood-onset systemic lupus erythematosus. Pediatr. Res. 96, 494–501 (2024).
Narendra, R. et al. Epigenetic attenuation of interferon signaling is associated with aging-related improvements in systemic lupus erythematosus. Sci. Transl. Med. 17, eadt5550 (2025).
Rees, F. et al. The incidence and prevalence of systemic lupus erythematosus in the UK, 1999-2012. Ann. Rheum. Dis. 75, 136–141 (2016).
Hiraki, L. T. et al. Ethnic differences in pediatric systemic lupus erythematosus. J. Rheumatol. 36, 2539–2546 (2009).
Massias, J. S. et al. Clinical and laboratory phenotypes in juvenile-onset systemic lupus erythematosus across ethnicities in the UK. Lupus 30, 597–607 (2021).
Levy, D. M. et al. Influence of ethnicity on childhood-onset systemic lupus erythematosus: results from a multiethnic multicenter Canadian cohort. Arthritis Care Res. 65, 152–160 (2013).
Demkova, K., Morris, D. L. & Vyse, T. J. Genetics of SLE: does this explain susceptibility and severity across racial groups? Rheumatology 62, i15–i21 (2023).
Arnaud, L. et al. Burden of systemic lupus erythematosus in clinical practice: baseline data from the SLE Prospective Observational Cohort Study (SPOCS) by interferon gene signature. Lupus Sci. Med. 10, e001032 (2023).
Robinson, G. A. et al. Editorial: sex bias in autoimmunity: from animal models to clinical research and applications. Front. Med. 9, 1112966 (2022).
Robinson, G. A. et al. Sex differences in lipid metabolism: implications for systemic lupus erythematosus and cardiovascular disease risk. Front. Med. 9, 914016 (2022).
Peckham, H. et al. Gender-diverse inclusion in immunological research: benefits to science and health. Front. Med. 9, 909789 (2022).
Robinson, G. A. et al. Investigating sex differences in T regulatory cells from cisgender and transgender healthy individuals and patients with autoimmune inflammatory disease: a cross-sectional study. Lancet Rheumatol. 4, e710–e724 (2022).
Peckham, H. et al. Estrogen influences class-switched memory B cell frequency only in humans with two X chromosomes. J. Exp. Med. 222, e20241253 (2025).
Webb, K. et al. Sex and pubertal differences in the type 1 interferon pathway associate with both X chromosome number and serum sex hormone concentration. Front. Immunol. 9, 3167 (2018).
Lakshmikanth, T. et al. Immune system adaptation during gender-affirming testosterone treatment. Nature 633, 155–164 (2024).
Renau, A. I. & Isenberg, D. A. Male versus female lupus: a comparison of ethnicity, clinical features, serology and outcome over a 30 year period. Lupus 21, 1041–1048 (2012).
Vieira, A. A. et al. Female-bias in systemic lupus erythematosus: how much is the X chromosome to blame? Biol. Sex. Differ. 15, 76 (2024).
Syrett, C. M. & Anguera, M. C. When the balance is broken: X-linked gene dosage from two X chromosomes and female-biased autoimmunity. J. Leukoc. Biol. 106, 919–932 (2019).
Liu, K. et al. X chromosome dose and sex bias in autoimmune diseases: increased prevalence of 47,XXX in systemic lupus erythematosus and Sjögren’s syndrome. Arthritis Rheumatol. 68, 1290–1300 (2016).
Crawford, J. D. et al. The XIST lncRNA is a sex-specific reservoir of TLR7 ligands in SLE. JCI Insight 8, e169344 (2023).
Robinson, G. A. et al. Disease-associated and patient-specific immune cell signatures in juvenile-onset systemic lupus erythematosus: patient stratification using a machine-learning approach. Lancet Rheumatol. 2, e485–e496 (2020).
Nehar-Belaid, D. et al. Mapping systemic lupus erythematosus heterogeneity at the single-cell level. Nat. Immunol. 21, 1094–1106 (2020).
Perez, R. K. et al. Single-cell RNA-seq reveals cell type-specific molecular and genetic associations to lupus. Science 376, eabf1970 (2022).
Radziszewska, A. et al. Type I interferon and mitochondrial dysfunction are associated with dysregulated cytotoxic CD8+ T cell responses in juvenile systemic lupus erythematosus. Clin. Exp. Immunol. 219, uxae127 (2025).
Baxter, R. M. et al. Expansion of extrafollicular B and T cell subsets in childhood-onset systemic lupus erythematosus. Front. Immunol. 14, 1208282 (2023).
Danaher, P. et al. Childhood-onset lupus nephritis is characterized by complex interactions between kidney stroma and infiltrating immune cells. Sci. Transl. Med. 16, eadl1666 (2024).
Arazi, A. et al. The immune cell landscape in kidneys of patients with lupus nephritis. Nat. Immunol. 20, 902–914 (2019).
Banchereau, R. et al. Personalized immunomonitoring uncovers molecular networks that stratify lupus patients. Cell 165, 551–565 (2016).
Ma, J. et al. Construction of molecular subgroups in childhood systemic lupus erythematosus using bioinformatics. Medicine 101, e32274 (2022).
Wahadat, M. J. et al. Targeted multiomics in childhood-onset SLE reveal distinct biological phenotypes associated with disease activity: results from an explorative study. Lupus Sci. Med. 10, e000799 (2023).
Psarras, A., Wittmann, M. & Vital, E. M. Emerging concepts of type I interferons in SLE pathogenesis and therapy. Nat. Rev. Rheumatol. 18, 575–590 (2022).
Natoli, V. et al. Elevated serum interferon-α2 associates with activity and flare risk in Juvenile-onset systemic lupus erythematosus. Rheumatology 64, 3938–3946 (2025).
Wahadat, M. J. et al. Serum IFNα2 levels are associated with disease activity and outperform IFN-I gene signature in a longitudinal childhood-onset SLE cohort. Rheumatology 62, 2872–2879 (2023).
Wahadat, M. J. et al. Gene signature fingerprints stratify SLE patients in groups with similar biological disease profiles: a multicentre longitudinal study. Rheumatology 61, 4344–4354 (2022).
Nombel, A. et al. Profiling type I and II interferon responses reveals distinct subgroups of pediatric patients with autoinflammatory disorders. J. Allergy Clin. Immunol. Glob. 4, 100450 (2025).
Bradford, H. F. et al. Inactive disease in patients with lupus is linked to autoantibodies to type I interferons that normalize blood IFNα and B cell subsets. Cell Rep. Med. 4, 100894 (2023).
Macêdo, M. B. et al. Anti-mitochondrial antibodies as markers of disease activity in childhood-onset systemic lupus erythematosus: a longitudinal cohort study. Rheumatology 64, 5096–5100 (2025).
Caielli, S. et al. Oxidized mitochondrial nucleoids released by neutrophils drive type I interferon production in human lupus. J. Exp. Med. 213, 697–713 (2016).
Ciurtin, C. Potential relevance of type I interferon-related biomarkers for the management of polygenic autoimmune rheumatic diseases with childhood onset. Clin. Rheumatol. 42, 1733–1736 (2023).
Oni, L. et al. Kidney outcomes for children with lupus nephritis. Pediatr. Nephrol. 36, 1377–1385 (2021).
Greenan-Barrett, J. et al. Biomarkers associated with organ-specific involvement in juvenile systemic lupus erythematosus. Int. J. Mol. Sci. 22, 7619 (2021).
Soliman, S. A. et al. Urine ALCAM, PF4 and VCAM-1 surpass conventional metrics in identifying nephritis disease activity in childhood-onset systemic lupus erythematosus. Front. Immunol. 13, 885307 (2022).
Li, Y. et al. Proximity extension assay proteomics and renal single cell transcriptomics uncover novel urinary biomarkers for active lupus nephritis. J. Autoimmun. 143, 103165 (2024).
Ciurtin, C. et al. Cardiovascular risk in young people with childhood onset systemic lupus erythematosus. Lancet Rheumatol. 6, e258–e263 (2024).
Ciurtin, C. et al. Comorbidity in young patients with juvenile systemic lupus erythematosus: how can we improve management? Clin. Rheumatol. 41, 961–964 (2022).
Robinson, G. A. et al. Metabolomics defines complex patterns of dyslipidaemia in juvenile-SLE patients associated with inflammation and potential cardiovascular disease risk. Metabolites 12, 3 (2022).
Jury, E. C. et al. Systemic lupus erythematosus patients have unique changes in serum metabolic profiles across age associated with cardiometabolic risk. Rheumatology 63, 2741–2753 (2023).
Robinson, G. et al. Lipid metabolism in autoimmune rheumatic disease: implications for modern and conventional therapies. J. Clin. Invest. 132, e148552 (2022).
Schanberg, L. E. et al. Premature atherosclerosis in pediatric systemic lupus erythematosus: risk factors for increased carotid intima-media thickness in the atherosclerosis prevention in pediatric lupus erythematosus cohort. Arthritis Rheum. 60, 1496–1507 (2009).
Petri, M. A. et al. Lupus Atherosclerosis Prevention Study (LAPS). Ann. Rheum. Dis. 70, 760–765 (2011).
Peng, J. et al. Atherosclerosis progression in the APPLE trial can be predicted in young people with juvenile-onset systemic lupus erythematosus using a novel lipid metabolomic signature. Arthritis Rheumatol. 76, 455–468 (2024).
Robinson, G. A. et al. Increased apolipoprotein-B:A1 ratio predicts cardiometabolic risk in patients with juvenile onset SLE. EBioMedicine 65, 103243 (2021).
Ciurtin, C. et al. Investigation of the performance of validated cardiovascular risk scores in a global (UK/US) cohort of young people with childhood-onset systemic lupus erythematosus. Lupus Sci. Med. 11, e001194 (2024).
Woodridge, L. et al. Subclinical atherosclerosis risk can be predicted in female patients with systemic lupus erythematosus using metabolomic signatures: an observational study. J. Am. Heart Assoc. 14, e036507 (2025).
Rubinstein, T. B., Putterman, C. & Goilav, B. Biomarkers for CNS involvement in pediatric lupus. Biomark. Med. 9, 545–558 (2015).
van der Heijden, H. et al. Implications of inflammatory processes on a developing central nervous system in childhood-onset systemic lupus erythematosus. Arthritis Rheumatol. 76, 332–344 (2024).
Moraitis, E. et al. Aquaporin-4 IgG antibody-related disorders in patients with juvenile systemic lupus erythematosus. Lupus 28, 1243–1249 (2019).
Brunner, H. I. et al. Blood-based candidate biomarkers of the presence of neuropsychiatric systemic lupus erythematosus in children. Lupus Sci. Med. 1, e000038 (2014).
Kammeyer, R. et al. Blood-based biomarkers of neuronal and glial injury in active major neuropsychiatric systemic lupus erythematosus. Lupus 33, 1116–1129 (2024).
Muscal, E. & Brey, R. L. Neurologic manifestations of systemic lupus erythematosus in children and adults. Neurol. Clin. 28, 61–73 (2010).
Valdés Cabrera, D. et al. Effects of systemic lupus erythematosus on the brain: a systematic review of structural MRI findings and their relationships with cognitive dysfunction. Lupus Sci. Med. 11, e001214 (2024).
Frittoli, R. B. et al. Axonal dysfunction is associated with interferon-γ levels in childhood-onset systemic lupus erythematosus: a multivoxel magnetic resonance spectroscopy study. Rheumatology 61, 1529–1537 (2022).
DiFrancesco, M. W. et al. Functional neuronal network activity differs with cognitive dysfunction in childhood-onset systemic lupus erythematosus. Arthritis Res. Ther. 15, R40 (2013).
Vega-Fernandez, P. et al. Cognitive performance scores for the pediatric automated neuropsychological assessment metrics in childhood-onset systemic lupus erythematosus. Arthritis Care Res. 67, 1119–1127 (2015).
Askanase, A. et al. New and future therapies: changes in the therapeutic armamentarium for SLE. Best Pract. Res. Clin. Rheumatol. 37, 101865 (2023).
Bruce, I. N. Health inequalities and systemic lupus erythematosus: a global challenge. Rheumatology 62, i1–i3 (2023).
Ekinci, Z. & Ozturk, K. Systemic lupus erythematosus with C1q deficiency: treatment with fresh frozen plasma. Lupus 27, 134–138 (2018).
Akbar, L., Alsagheir, R. & Al-Mayouf, S. M. Efficacy of a sequential treatment by belimumab in monogenic systemic lupus erythematosus. Eur. J. Rheumatol. 7, 184–189 (2020).
Matsumura, R. et al. Bone marrow transplantation from a human leukocyte antigen-mismatched unrelated donor in a case with C1q deficiency associated with refractory systemic lupus erythematosus. Int. J. Hematol. 113, 302–307 (2021).
Tilstra, J. S. et al. B cell-intrinsic Myd88 regulates disease progression in murine lupus. J. Exp. Med. 220, e20230263 (2023).
Cenac, C., Ducatez, M. F. & Guery, J. C. Hydroxychloroquine inhibits proteolytic processing of endogenous TLR7 protein in human primary plasmacytoid dendritic cells. Eur. J. Immunol. 52, 54–61 (2022).
Doroudchi, A. & Butte, M. First reported use of anifrolumab to treat a monogenic interferonopathy (DNASE2 loss of function). Clin. Immunol. 250, 109593 (2023).
Felten, R., Scherlinger, M., Mertz, P., Chasset, F. & Arnaud, L. New biologics and targeted therapies in systemic lupus: from new molecular targets to new indications. A systematic review. Jt Bone Spine 90, 105523 (2023).
Wu, J. et al. Case report: JAK1/2 inhibition with baricitinib in the treatment of STING-associated vasculopathy with onset in infancy. Pediatr. Rheumatol. Online J. 21, 131 (2023).
Decout, A. et al. The cGAS–STING pathway as a therapeutic target in inflammatory diseases. Nat. Rev. Immunol. 21, 548–569 (2021).
Su, G. et al. Analysis of five cases of monogenic lupus related to primary immunodeficiency diseases. Inflamm. Res. 70, 1211–1216 (2021).
Lei, L. et al. Successful use of ofatumumab in two cases of early-onset juvenile SLE with thrombocytopenia caused by a mutation in protein kinase C δ. Pediatr. Rheumatol. Online J. 16, 61 (2018).
Moreews, M. et al. mTOR activation underlies enhanced B cell proliferation and autoimmunity in PrkcdG510S/G510S mice. J. Immunol. 210, 1209–1221 (2023).
Elhani, I. et al. A20 haploinsufficiency: a systematic review of 177 cases. J. Investig. Dermatol. 144, 1282–1294.e8 (2024).
Liu, J. et al. Haploinsufficiency of A20 in a Chinese child caused by loss-of-function mutations in TNFAIP3: a case report and review of the literature. Front. Pediatrics 10, 990008 (2023).
Brunner, H. I. et al. Safety and efficacy of intravenous belimumab in children with systemic lupus erythematosus: results from a randomised, placebo-controlled trial. Ann. Rheum. Dis. 79, 1340–1348 (2020).
Md Yusof, M. Y. et al. Management and treatment of children, young people and adults with systemic lupus erythematosus: British Society for Rheumatology guideline scope. Rheumatol. Adv. Pract. 7, rkad093 (2023).
Sammaritano, L. R. et al. 2024 American College of Rheumatology (ACR) guideline for the screening, treatment, and management of lupus nephritis. Arthritis Rheumatol. 77, 1115–1135 (2025).
Fanouriakis, A. et al. EULAR recommendations for the management of systemic lupus erythematosus: 2023 update. Ann. Rheum. Dis. 83, 15–29 (2024).
Lin, T. W. et al. Rituximab as an effective add-on maintenance therapy for disease activities in childhood-onset systemic lupus erythematosus. Lupus Sci. Med. 11, e000987 (2024).
Cognard, J. et al. Indications, efficacy, safety of rituximab in childhood-onset systemic lupus erythematosus: a retrospective study of the JIR cohort. Rheumatology 64, 4921–4929 (2025).
Tambralli, A. et al. Safety and efficacy of rituximab in childhood-onset systemic lupus erythematosus and other rheumatic diseases. J. Rheumatol. 42, 541–546 (2015).
Li, H. et al. Efficacy and safety of belimumab combined with the standard regimen in treating children with lupus nephritis. Eur. J. Pediatr. 183, 3987–3995 (2024).
Cinar, O. K. et al. Ofatumumab use in juvenile systemic lupus erythematosus: a single centre experience. Lupus 30, 527–530 (2021).
Tanaka, H., Aizawa, T. & Endo, M. Long-term outcome of tacrolimus-based immunosuppressive treatment for patients with paediatric-onset lupus nephritis. Nephrology 29, 901–908 (2024).
Fasano, S. et al. Precision medicine in systemic lupus erythematosus. Nat. Rev. Rheumatol. 19, 331–342 (2023).
He, X. et al. Treatment of two pediatric patients with refractory systemic lupus erythematosus using CD19-targeted CAR T-cells. Autoimmun. Rev. 24, 103692 (2025).
Wobma, H. et al. CAR T cell therapy for children with rheumatic disease: the time is now. Nat. Rev. Rheumatol. 21, 494–506 (2025).
Chalhoub, N. E. et al. International consensus for the dosing of corticosteroids in childhood-onset systemic lupus erythematosus with proliferative lupus nephritis. Arthritis Rheumatol. 74, 263–273 (2022).
Smith, E. M. D. et al. Towards development of treat to target (T2T) in childhood-onset systemic lupus erythematosus: PReS-endorsed overarching principles and points-to-consider from an international task force. Ann. Rheum. Dis. 82, 788–798 (2023).
Franklyn, K. et al. Definition and initial validation of a Lupus Low Disease Activity State (LLDAS). Ann. Rheum. Dis. 75, 1615–1621 (2016).
Smith, E. M. D. et al. PReS-endorsed international childhood lupus T2T task force definition of childhood lupus low disease activity state (cLLDAS). Clin. Immunol. 250, 109296 (2023).
van Vollenhoven, R. F. et al. 2021 DORIS definition of remission in SLE: final recommendations from an international task force. Lupus Sci. Med. 8, e000538 (2021).
Soulsby, W. D. et al. Racial disparities and achievement of the low lupus disease activity state: a CARRA registry study. Arthritis Care Res. 77, 38–49 (2025).
Sadun, R. E. et al. Development of CARRA/PReS-endorsed consensus core and expanded datasets in childhood-onset systemic lupus erythematosus for international registry-based research. Ann. Rheum. Dis. 84, 158–168 (2025).
Ciurtin, C., Pineda-Torra, I., Jury, E. C. & Robinson, G. A. CD8+ T-cells in juvenile-onset SLE: from pathogenesis to comorbidities. Front. Med. 9, 904435 (2025).
Acknowledgements
G.A.R. was supported by an Arthritis UK Career Development Fellowship (22856). C.C. was supported by a National Institute of Heath Research (NIHR) University College London Hospital (UCLH) Biomedical Research Centre (ref. BRC4/III/CC) grant. A.K. was supported by a Canadian Institutes of Health Research Canada Research Chair Tier 2 and Lupus Research Alliance Career Development Award.
Author information
Authors and Affiliations
Contributions
G.A.R., C.C., D.A.I. and A.B. researched data for the article. G.A.R., C.C. and D.A.I. contributed substantially to discussion of the content. All authors wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks Claudia Saad-Magalhães, Sylvia Kamphuis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Robinson, G.A., Knight, A., Tucker, L.B. et al. Insights into the pathogenesis of childhood-onset SLE in the past decade. Nat Rev Rheumatol 22, 26–41 (2026). https://doi.org/10.1038/s41584-025-01321-x
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41584-025-01321-x