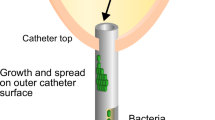
Abstract
Urinary tract infections are one of the most common infections, accounting for ~400 million diagnoses per year worldwide. Uncomplicated urinary tract infections (uUTIs) occur in healthy individuals with no structural or functional abnormalities of the urinary system and primarily affect women. Catheter-associated urinary tract infections (CAUTIs) are a type of complicated UTI affecting patients who have a urinary catheter in place, often hospitalized patients or patients with conditions that prevent them from urinating naturally. Both infections share common symptoms, diagnostics and treatment options but also differ greatly in pathophysiology, aetiology, risk factors and comorbidities. These differences could explain why antibiotic treatments — which generally lead to positive outcomes in patients with uUTIs — often fail in patients with CAUTIs. Understanding these differences could guide evidence-based insights into why treatments for CAUTIs should be different from those for uUTIs, specifically, by modifying catheters, which initiate the damage-induced segue for UTIs.
Key points
-
Urinary tract infections (UTIs) are an extremely common diagnosis in the USA and globally.
-
Uncomplicated and catheter-associated UTIs differ greatly in pathogen diversity, pathophysiology and propensity towards secondary bloodstream infections.
-
New treatments for catheter-associated UTIs should be specifically designed for these infections, rather than simply adapted from therapies for uncomplicated UTIs To reduce antibiotic resistance among urinary pathogens, modern treatments and preventative strategies can be designed to target specific mechanisms used by uropathogens to cause different types of UTI.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Tandogdu, Z. et al. Urosepsis 30-day mortality, morbidity, and their risk factors: SERPENS study, a prospective, observational multi-center study. World J. Urol. 42, 314 (2024).
Kuehn, B. M. Some progress in effort to reduce hospital-acquired infections. JAMA 311, 1488 (2014).
Klevens, R. M. et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public. Health Rep. 122, 160–166 (2007).
Foxman, B. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. North. Am. 28, 1–13 (2014).
Werneburg, G. T. Catheter-associated urinary tract infections: current challenges and future prospects. Res. Rep. Urol. 14, 109–133 (2022).
Yang, X. et al. Disease burden and long-term trends of urinary tract infections: a worldwide report. Front. Public. Health 10, 888205 (2022).
Flores-Mireles, A. L., Walker, J. N., Caparon, M. & Hultgren, S. J. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 13, 269–284 (2015).
Medina, M. & Castillo-Pino, E. An introduction to the epidemiology and burden of urinary tract infections. Ther. Adv. Urol. 11, 1756287219832172 (2019).
United States Center for Disease Control and Prevention. Urinary Tract Infections Basics. CDC https://www.cdc.gov/uti/about/index.html (2024).
Czajkowski, K., Bros-Konopielko, M. & Teliga-Czajkowska, J. Urinary tract infection in women. Prz. Menopauzalny 20, 40–47 (2021).
Pujades-Rodriguez, M., West, R. M., Wilcox, M. H. & Sandoe, J. Lower urinary tract infections: management, outcomes and risk factors for antibiotic re-prescription in primary care. EClinicalMedicine 14, 23–31 (2019).
Koh, S. W. C. et al. Antibiotic treatment failure of uncomplicated urinary tract infections in primary care. Antimicrob. Resist. Infect. Control. 12, 73 (2023).
McLellan, L. K. & Hunstad, D. A. Urinary tract infection: pathogenesis and outlook. Trends Mol. Med. 22, 946–957 (2016).
Flores-Mireles, A., Hreha, T. N. & Hunstad, D. A. Pathophysiology, treatment, and prevention of catheter-associated urinary tract infection. Top. Spinal Cord. Inj. Rehabil. 25, 228–240 (2019).
Wagenlehner, F. M. E. et al. Epidemiology, definition and treatment of complicated urinary tract infections. Nat. Rev. Urol. 17, 586–600 (2020).
Lo, E. et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect. Control. Hosp. Epidemiol. 35, 464–479 (2014).
Jacobsen, S. M., Stickler, D. J., Mobley, H. L. & Shirtliff, M. E. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin. Microbiol. Rev. 21, 26–59 (2008).
Langford, B. J. et al. Antibiotic selection and duration for catheter-associated urinary tract infection in non-hospitalized older adults: a population-based cohort study. Antimicrob. Steward. Healthc. Epidemiol. 3, e132 (2023).
Paul, R. State of the globe: rising antimicrobial resistance of pathogens in urinary tract infection. J. Glob. Infect. Dis. 10, 117–118 (2018).
Haider, M.Z. & Annamaraju, P. Bladder Catheterization. StatPearls [Internet] https://www.ncbi.nlm.nih.gov/books/NBK560748 (updated 9 August 2023).
Wilson Dib, R. et al. The impact of the COVID-19 pandemic on hospital-acquired infections at a comprehensive cancer center. Am. J. Infect. Control. 51, 1302–1308 (2023).
United States Center for Disease Control and Prevention. COVID-19 Impact on Healthcare-associated Infections. CDC https://www.cdc.gov/healthcare-associated-infections/php/data/covid-impact.html (2024).
Parker, D. et al. Nursing interventions to reduce the risk of catheter-associated urinary tract infection. Part 1: catheter selection. J. Wound Ostomy Cont. Nurs. 36, 23–34 (2009).
Jarrell, A. S. et al. Short-duration treatment for catheter-associated urinary tract infections in critically ill trauma patients. J. Trauma. Acute Care Surg. 79, 649–653 (2015).
Willson, M. et al. Nursing interventions to reduce the risk of catheter-associated urinary tract infection: part 2: staff education, monitoring, and care techniques. J. Wound Ostomy Cont. Nurs. 36, 137–154 (2009).
Maki, D. G. & Tambyah, P. A. Engineering out the risk for infection with urinary catheters. Emerg. Infect. Dis. 7, 342–347 (2001).
Kunin, C. M., Douthitt, S., Dancing, J., Anderson, J. & Moeschberger, M. The association between the use of urinary catheters and morbidity and mortality among elderly patients in nursing homes. Am. J. Epidemiol. 135, 291–301 (1992).
Dreger, N. M., Degener, S., Ahmad-Nejad, P., Wobker, G. & Roth, S. Urosepsis — etiology, diagnosis, and treatment. Dtsch. Arztebl Int. 112, 837–847 (2015). quiz 848.
Rosenthal, V. D. et al. Time-dependent analysis of length of stay and mortality due to urinary tract infections in ten developing countries: INICC findings. J. Infect. 62, 136–141 (2011).
Trautner, B. W. & Darouiche, R. O. Catheter-associated infections: pathogenesis affects prevention. Arch. Intern. Med. 164, 842–850 (2004).
Tambyah, P. A. & Oon, J. Catheter-associated urinary tract infection. Curr. Opin. Infect. Dis. 25, 365–370 (2012).
Warren, J. W. Catheter-associated urinary tract infections. Infect. Dis. Clin. North. Am. 11, 609–622 (1997).
Johnson, J. R., Kuskowski, M. A. & Wilt, T. J. Systematic review: antimicrobial urinary catheters to prevent catheter-associated urinary tract infection in hospitalized patients. Ann. Intern. Med. 144, 116–126 (2006).
Tambyah, P. A. & Maki, D. G. Catheter-associated urinary tract infection is rarely symptomatic — a prospective study of 1497 catheterized patients. Arch. Intern. Med. 160, 678–682 (2000).
Tambyah, P. A., Knasinski, V. & Maki, D. G. The direct costs of nosocomial catheter-associated urinary tract infection in the era of managed care. Infect. Control. Hosp. Epidemiol. 23, 27–31 (2002).
Morgan, D. J. et al. Does nonpayment for hospital-acquired catheter-associated urinary tract infections lead to overtesting and increased antimicrobial prescribing? Clin. Infect. Dis. 55, 923–929 (2012).
Gould, C. V. et al. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect. Control. Hosp. Epidemiol. 31, 319–326 (2010).
Ling, M. L. et al. APSIC guide for prevention of catheter associated urinary tract infections (CAUTIs). Antimicrobial Resistance Infect. Control. 12, 52 (2023).
Saint, S. et al. Preventing catheter-associated urinary tract infection in the United States: a national comparative study. JAMA Intern. Med. 173, 874–879 (2013).
Carter, A. E. et al. Evaluation of CAUTI-reduction strategies and associated reduction in CAUTI rate and catheter utilization at a large academic medical center. Am. J. Infect. Control. 51, S51 (2023).
Hayes, B. W. & Abraham, S. N. Innate immune responses to bladder infection. Microbiol. Spectr. https://doi.org/10.1128/microbiolspec.uti-0024-2016 (2016).
Ding, Y., Wang, H., Pu, S., Huang, S. & Niu, S. Resistance trends of Klebsiella pneumoniae causing urinary tract infections in Chongqing, 2011–2019. Infect. Drug. Resist. 14, 475–481 (2021).
Ronald, A. The etiology of urinary tract infection: traditional and emerging pathogens. Dis. Mon. 49, 71–82 (2003).
DeRosa, A. et al. Antimicrobial susceptibility trends for urinary isolates in the veteran population. Am. J. Infect. Control. 49, 576–581 (2021).
Schembri, M. A., Nhu, N. T. K. & Phan, M.-D. Gut–bladder axis in recurrent UTI. Nat. Microbiol. 7, 601–602 (2022).
Klein, R. D. & Hultgren, S. J. Urinary tract infections: microbial pathogenesis, host-pathogen interactions and new treatment strategies. Nat. Rev. Microbiol. 18, 211–226 (2020).
Guiton, P. S. et al. Combinatorial small-molecule therapy prevents uropathogenic Escherichia coli catheter-associated urinary tract infections in mice. Antimicrob. Agents Chemother. 56, 4738–4745 (2012).
Abraham, S. N. & Miao, Y. The nature of immune responses to urinary tract infections. Nat. Rev. Immunol. 15, 655–663 (2015).
Song, J. et al. A novel TLR4-mediated signaling pathway leading to IL-6 responses in human bladder epithelial cells. PLoS Pathog. 3, e60 (2007).
Ching, C. B. et al. Interleukin-6/Stat3 signaling has an essential role in the host antimicrobial response to urinary tract infection. Kidney Int. 93, 1320–1329 (2018).
Dixit, A. et al. Frontline science: proliferation of Ly6C+ monocytes during urinary tract infections is regulated by IL-6 trans-signaling. J. Leukoc. Biol. 103, 13–22 (2018).
Hunstad, D. A., Justice, S. S., Hung, C. S., Lauer, S. R. & Hultgren, S. J. Suppression of bladder epithelial cytokine responses by uropathogenic Escherichia coli. Infect. Immun. 73, 3999–4006 (2005).
Hilbert, D. W. et al. Uropathogenic Escherichia coli dominantly suppress the innate immune response of bladder epithelial cells by a lipopolysaccharide- and Toll-like receptor 4-independent pathway. Microbes Infect. 10, 114–121 (2008).
Yu, L. et al. Mucosal infection rewires TNFα signaling dynamics to skew susceptibility to recurrence. Elife 8, e46677 (2019).
Al Qahtani, M. et al. The incidence, clinical features and outcome of urinary tract infections in geriatric patients: a prospective longitudinal study. IJID Reg. 13, 100469 (2024).
O’Brien, V. P., Dorsey, D. A., Hannan, T. J. & Hultgren, S. J. Host restriction of Escherichia coli recurrent urinary tract infection occurs in a bacterial strain-specific manner. PLoS Pathog. 14, e1007457 (2018).
O’Brien, V. P. et al. A mucosal imprint left by prior Escherichia coli bladder infection sensitizes to recurrent disease. Nat. Microbiol. 2, 16196 (2016).
O’Brien, V. P., Hannan, T. J., Schaeffer, A. J. & Hultgren, S. J. Are you experienced? Understanding bladder innate immunity in the context of recurrent urinary tract infection. Curr. Opin. Infect. Dis. 28, 97–105 (2015).
Russell, S. K. et al. Uropathogenic Escherichia coli infection-induced epithelial trained immunity impacts urinary tract disease outcome. Nat. Microbiol. 8, 875–888 (2023).
Huang, X. Z., Zhu, L. B., Li, Z. R. & Lin, J. Bacterial colonization and intestinal mucosal barrier development. World J. Clin. Pediatr. 2, 46–53 (2013).
Hannan, T. J. et al. Inhibition of cyclooxygenase-2 prevents chronic and recurrent cystitis. EBioMedicine 1, 46–57 (2014).
Ebrahimzadeh, T. et al. Urinary prostaglandin E2 as a biomarker for recurrent UTI in postmenopausal women. Life Sci. Alliance 4, e202000948 (2021).
Bleidorn, J., Gagyor, I., Kochen, M. M., Wegscheider, K. & Hummers-Pradier, E. Symptomatic treatment (ibuprofen) or antibiotics (ciprofloxacin) for uncomplicated urinary tract infection? — results of a randomized controlled pilot trial. BMC Med. 8, 30 (2010).
Tenke, P., Mezei, T., Bőde, I. & Köves, B. Catheter-associated urinary tract infections. Eur. Urol. Suppl. 16, 138–143 (2017).
Barford, J. M., Hu, Y., Anson, K. & Coates, A. R. A biphasic response from bladder epithelial cells induced by catheter material and bacteria: an in vitro study of the pathophysiology of catheter related urinary tract infection. J. Urol. 180, 1522–1526 (2008).
Agra Leite, C. & Darbellay Farhoumand, P. [The forgotten urinary catheter]. Rev. Med. Suisse 19, 589–590 (2023).
Andersen, M. J. et al. Inhibiting host-protein deposition on urinary catheters reduces associated urinary tract infections. Elife 11, e75798 (2022).
Molina, J. J. et al. Fibrinolytic-deficiencies predispose hosts to septicemia from a catheter-associated UTI. Nat. Commun. 15, 2704 (2024).
Liu, H. Y., Prentice, E. L. & Webber, M. A. Mechanisms of antimicrobial resistance in biofilms. NPJ Antimicrob. Resist. 2, 27 (2024).
Trautner, B. W. & Darouiche, R. O. Role of biofilm in catheter-associated urinary tract infection. Am. J. Infect. Control. 32, 177–183 (2004).
Gunardi, W. D. et al. Biofilm-producing bacteria and risk factors (gender and duration of catheterization) characterized as catheter-associated biofilm formation. Int. J. Microbiol. 2021, 8869275 (2021).
Wilks, S. A., Koerfer, V. V., Prieto, J. A., Fader, M. & Keevil, C. W. Biofilm development on urinary catheters promotes the appearance of viable but nonculturable bacteria. mBio 12, e03584–20 (2021).
Ramadan, R., Omar, N., Dawaba, M. & Moemen, D. Bacterial biofilm dependent catheter associated urinary tract infections: characterization, antibiotic resistance pattern and risk factors. Egypt. J. Basic. Appl. Sci. 8, 64–74 (2021).
Soto, S. M. Importance of biofilms in urinary tract infections: new therapeutic approaches. Adv. Biol. 2014, 543974 (2014).
Andersen, M. J. & Flores-Mireles, A. L. Urinary catheter coating modifications: the race against catheter-associated infections. Coatings 10, 23 (2020).
Flores-Mireles, A. L. et al. Antibody-based therapy for enterococcal catheter-associated urinary tract infections. mBio 7, e01653–16 (2016).
La Bella, A. A. et al. The catheterized bladder environment promotes Efg1- and Als1-dependent Candida albicans infection. Sci. Adv. 9, eade7689 (2023).
Flores-Mireles, A. L. et al. Fibrinogen release and deposition on urinary catheters placed during urological procedures. J. Urol. 196, 416–421 (2016).
Chaudhry, R., Killeen, R. B. & Babiker H., M. Physiology, Coagulation Pathways. StatPearls [Internet] https://www.ncbi.nlm.nih.gov/books/NBK482253/ (updated 2 June 2025).
Cheung, A. L. & Fischetti, V. A. The role of fibrinogen in staphylococcal adherence to catheters in vitro. J. Infect. Dis. 161, 1177–1186 (1990).
Bertoglio, F. et al. Antibodies to coagulase of Staphylococcus aureus crossreact to Efb and reveal different binding of shared fibrinogen binding repeats. Front. Immunol. 14, 1221108 (2023).
Li, Q. et al. Fibronectin-/fibrinogen-binding protein (FBPS) is not a critical virulence factor for the Streptococcus suis serotype 2 strain ZY05719. Vet. Microbiol. 208, 38–46 (2017).
Lapschies, A. M. et al. The type-2 Streptococcus canis M protein SCM-2 binds fibrinogen and facilitates antiphagocytic properties. Front. Microbiol. 14, 1228472 (2023).
National Healthcare Safety Network. Device Associated Module: Urinary Tract Infection (Catheter-Associated Urinary Tract Infection [CAUTI] and Non-Catheter-Associated Urinary Tract Infection [UTI]) Events. CDC https://www.cdc.gov/nhsn/pdfs/pscmanual/7psccauticurrent.pdf (2021).
Holroyd-Leduc, J. M. et al. Risk factors for indwelling urinary catheterization among older hospitalized patients without a specific medical indication for catheterization. J. Patient Saf. 1, 201–207 (2005).
Kaur, R. & Kaur, R. Symptoms, risk factors, diagnosis and treatment of urinary tract infections. Postgrad. Med. J. 97, 803–812 (2020).
Ambite, I. et al. Molecular determinants of disease severity in urinary tract infection. Nat. Rev. Urol. 18, 468–486 (2021).
Al Lawati, H., Blair, B. M. & Larnard, J. Urinary tract infections: core curriculum 2024. Am. J. Kidney Dis. 83, 90–100 (2024).
Hay, A. D. et al. The diagnosis of urinary tract infection in young children (DUTY): a diagnostic prospective observational study to derive and validate a clinical algorithm for the diagnosis of urinary tract infection in children presenting to primary care with an acute illness. Health Technol. Assess. 20, 1–294 (2016).
Walker, J. N. et al. High-resolution imaging reveals microbial biofilms on patient urinary catheters despite antibiotic administration. World J. Urol. 38, 2237–2245 (2020).
Nye, T. M. et al. Microbial co-occurrences on catheters from long-term catheterized patients. Nat. Commun. 15, 61 (2024).
Gaston, J. R. et al. Enterococcus faecalis polymicrobial interactions facilitate biofilm formation, antibiotic recalcitrance, and persistent colonization of the catheterized urinary tract. Pathogens 9, 835 (2020).
Armbruster, C. E., Brauer, A. L., Humby, M. S., Shao, J. & Chakraborty, S. Prospective assessment of catheter-associated bacteriuria clinical presentation, epidemiology, and colonization dynamics in nursing home residents. JCI Insight 6, e144775 (2021).
Armbruster, C. E., Prenovost, K., Mobley, H. L. & Mody, L. How often do clinically diagnosed catheter-associated urinary tract infections in nursing homes meet standardized criteria? J. Am. Geriatr. Soc. 65, 395–401 (2017).
Qin, X. et al. Efficacy of expanded periurethral cleansing in reducing catheter-associated urinary tract infection in comatose patients: a randomized controlled clinical trial. Crit. Care 28, 162 (2024).
Azevedo, A. S., Almeida, C., Melo, L. F. & Azevedo, N. F. Impact of polymicrobial biofilms in catheter-associated urinary tract infections. Crit. Rev. Microbiol. 43, 423–439 (2017).
Melzer, M. & Welch, C. Outcomes in UK patients with hospital-acquired bacteraemia and the risk of catheter-associated urinary tract infections. Postgrad. Med. J. 89, 329–334 (2013).
Wagenlehner, F. M. et al. Diagnosis and management for urosepsis. Int. J. Urol. 20, 963–970 (2013).
Nasr, A. State of the globe: catheterizations continue to cultivate urinary infections. J. Glob. Infect. Dis. 2, 81–82 (2010).
Trautner, B. W. et al. A hospital-site controlled intervention using audit and feedback to implement guidelines concerning inappropriate treatment of catheter-associated asymptomatic bacteriuria. Implement. Sci. 6, 41 (2011).
Cope, M. et al. Inappropriate treatment of catheter-associated asymptomatic bacteriuria in a tertiary care hospital. Clin. Infect. Dis. 48, 1182–1188 (2009).
Kizilbash, Q. F., Petersen, N. J., Chen, G. J., Naik, A. D. & Trautner, B. W. Bacteremia and mortality with urinary catheter-associated bacteriuria. Infect. Control. Hosp. Epidemiol. 34, 1153–1159 (2013).
Flores-Mireles, A. L., Pinkner, J. S., Caparon, M. G. & Hultgren, S. J. EbpA vaccine antibodies block binding of Enterococcus faecalis to fibrinogen to prevent catheter-associated bladder infection in mice. Sci. Transl. Med. 6, 254ra127 (2014).
Ponnuraj, K. et al. A “dock, lock, and latch” structural model for a staphylococcal adhesin binding to fibrinogen. Cell 115, 217–228 (2003).
Thomas, S. et al. The complex fibrinogen interactions of the Staphylococcus aureus coagulases. Front. Cell Infect. Microbiol. 9, 106 (2019).
Negron, O. et al. Fibrin(ogen) engagement of S. aureus promotes the host antimicrobial response and suppression of microbe dissemination following peritoneal infection. PLoS Pathog. 18, e1010227 (2022).
Negron, O. et al. Fibrinogen γ’ promotes host survival during Staphylococcus aureus septicemia in mice. J. Thromb. Haemost. 21, 2277–2290 (2023).
Walker, J. N. et al. Catheterization alters bladder ecology to potentiate Staphylococcus aureus infection of the urinary tract. Proc. Natl Acad. Sci. USA 114, E8721–E8730 (2017).
Heintz, B. H., Halilovic, J. & Christensen, C. L. Vancomycin-resistant enterococcal urinary tract infections. Pharmacotherapy 30, 1136–1149 (2010).
Jayakumar, S. et al. Short term urinary catheterized patients in intensive care unit (ICU) — a need to screen. J. Commun. Dis. 43, 25–30 (2011).
Xu, W. et al. Host and bacterial proteases influence biofilm formation and virulence in a murine model of enterococcal catheter-associated urinary tract infection. NPJ Biofilms Microbiomes 3, 28 (2017).
Di Venanzio, G. et al. Urinary tract colonization is enhanced by a plasmid that regulates uropathogenic Acinetobacter baumannii chromosomal genes. Nat. Commun. 10, 2763 (2019).
Tamadonfar, K. O. et al. Structure–function correlates of fibrinogen binding by Acinetobacter adhesins critical in catheter-associated urinary tract infections. Proc. Natl Acad. Sci. USA 120, e2212694120 (2023).
Hazen, J. E., Di Venanzio, G., Hultgren, S. J. & Feldman, M. F. Catheterization of mice triggers resurgent urinary tract infection seeded by a bladder reservoir of Acinetobacter baumannii. Sci. Transl. Med. 15, eabn8134 (2023).
Kalas, V. et al. Evolutionary fine-tuning of conformational ensembles in FimH during host-pathogen interactions. Sci. Adv. 3, e1601944 (2017).
Adamczyk, B., Struwe, W. B., Ercan, A., Nigrovic, P. A. & Rudd, P. M. Characterization of fibrinogen glycosylation and its importance for serum/plasma N-glycome analysis. J. Proteome Res. 12, 444–454 (2013).
Alfouzan, W. A. & Dhar, R. Candiduria: evidence-based approach to management, are we there yet? J. Mycol. Med. 27, 293–302 (2017).
Fisher, J. F. Candiduria: when and how to treat it. Curr. Infect. Dis. Rep. 2, 523–530 (2000).
Hollenbach, E. To treat or not to treat — critically ill patients with candiduria. Mycoses 51, 12–24 (2008).
Sobel, J. D., Fisher, J. F., Kauffman, C. A. & Newman, C. A. Candida urinary tract infections — epidemiology. Clin. Infect. Dis. 52, S433–S436 (2011).
Jain, M. et al. Candiduria in catheterized intensive care unit patients: emerging microbiological trends. Indian J. Pathol. Microbiol. 54, 552–555 (2011).
Gharaghani, M., Taghipour, S., Halvaeezadeh, M. & Mahmoudabadi, A. Z. Candiduria; a review article with specific data from Iran. Turk. J. Urol. 44, 445–452 (2018).
Rishpana, M. S. & Kabbin, J. S. Candiduria in catheter associated urinary tract infection with special reference to biofilm production. J. Clin. Diagn. Res. 9, DC11–DC13 (2015).
Goetz, L. L., Howard, M., Cipher, D. & Revankar, S. G. Occurrence of candiduria in a population of chronically catheterized patients with spinal cord injury. Spinal Cord. 48, 51–54 (2010).
Parums, D. V. Editorial: the World Health Organization (WHO) fungal priority pathogens list in response to emerging fungal pathogens during the COVID-19 pandemic. Med. Sci. Monit. 28, e939088 (2022).
Organization, W. H. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. (World Health Organization, 2022).
Griffith, N. & Danziger, L. Candida auris urinary tract infections and possible treatment. Antibiotics 9, 898 (2020).
Padawer, D. et al. Catheter-associated candiduria: risk factors, medical interventions, and antifungal susceptibility. Am. J. Infect. Control. 43, e19–e22 (2015).
Álvarez-Lerma, F. et al. Candiduria in critically ill patients admitted to intensive care medical units. Intensive Care Med. 29, 1069–1076 (2003).
Walder, B. & Tramer, M. R. Analgesia and sedation in critically ill patients. Swiss Med. Wkly. 134, 333–346 (2004).
Walker, J. N. et al. High-resolution imaging reveals microbial biofilms on patient urinary catheters despite antibiotic administration. World J. Urol. 38, 2237–2245 (2019).
Kojic, E. M. & Darouiche, R. O. Candida infections of medical devices. Clin. Microbiol. Rev. 17, 255–267 (2004).
Azmy, M., Nawar, N., Mohiedden, M. & Warille, L. Electron microscopic assay of bacterial biofilm formed on indwelling urethral catheters. J. Egypt. Soc. Parasitol. 46, 475–484 (2016).
Fisher, J. F., Kavanagh, K., Sobel, J. D., Kauffman, C. A. & Newman, C. A. Candida urinary tract infection: pathogenesis. Clin. Infect. Dis. 52, S437–S451 (2011).
Walker, J. N. et al. High-resolution imaging reveals microbial biofilms on patient urinary catheters despite antibiotic administration. World J. Urol. 38, 2237–2245 (2018). 2020 Sep.
Zeller, I. et al. Detection of fungal pathogens by a new broad range real-time PCR assay targeting the fungal ITS2 region. J. Med. Microbiol. 66, 1383–1392 (2017).
Gajdacs, M., Doczi, I., Abrok, M., Lazar, A. & Burian, K. Epidemiology of candiduria and Candida urinary tract infections in inpatients and outpatients: results from a 10-year retrospective survey. Cent. European J. Urol. 72, 209–214 (2019).
He, Z. et al. Candiduria in hospitalized patients: an investigation with the Sysmex UF-1000i urine analyzer. PeerJ 7, e6935 (2019).
Li, H. et al. Interactions between Candida albicans and the resident microbiota. Front. Microbiol. 13, 930495 (2022).
Bouza, E. & Munoz, P. Epidemiology of candidemia in intensive care units. Int. J. Antimicrob. Agents 32, S87–S91 (2008).
Epelbaum, O. & Chasan, R. Candidemia in the intensive care unit. Clin. Chest Med. 38, 493–509 (2017).
Siegman-Igra, Y. The significance of urine culture with mixed flora. Curr. Opin. Nephrol. Hypertens. 3, 656–659 (1994).
Rudman, D., Hontanosas, A., Cohen, Z. & Mattson, D. E. Clinical correlates of bacteremia in a veterans administration extended care facility. J. Am. Geriatr. Soc. 36, 726–732 (1988).
Lee, H. S. & Le, J. in Infectious Diseases, (eds Huang, V., et al.) 7–28. (American College of Clinical Pharmacology, 2018).
Yu, Y. et al. Urethral catheter biofilms reveal plasticity in bacterial composition and metabolism and withstand host immune defenses in hypoxic environment. Front. Med. 8, 667462 (2021).
Allkja, J., Goeres, D. M., Azevedo, A. S. & Azevedo, N. F. Interactions of microorganisms within a urinary catheter polymicrobial biofilm model. Biotechnol. Bioeng. 120, 239–249 (2023).
Al-Balawi, M. & Morsy, F. M. Enterococcus faecalis is a better competitor than other lactic acid bacteria in the initial colonization of colon of healthy newborn babies at first week of their life. Front. Microbiol. 11, 2017 (2020).
United States Center for Disease Control and Prevention. HAI Pathogens and Antimicrobial Resistance Report, 2018–2021. CDC https://www.cdc.gov/nhsn/hai-report/index.html (2023).
Sheerin, N. S. Urinary tract infection. Medicine 39, 384–389 (2011).
Komala, M. & Kumar, K. S. Urinary tract infection: causes, symptoms, diagnosis and its management. Indian J. Res. Pharm. Biotechnol. 1, 226 (2013).
Sheerin, N. S. & Glover, E. K. Urinary tract infection. Medicine 47, 546–550 (2019).
Danchaivijitr, S., Dhiraputra, C., Cherdrungsi, R., Jintanothaitavorn, D. & Srihapol, N. Catheter-associated urinary tract infection. J. Med. Assoc. Thai 88, 26–30 (2005).
Meddings, J., Reichert, H. & McMahon, L. F. Jr Challenges and proposed improvements for reviewing symptoms and catheter use to identify national healthcare safety network catheter-associated urinary tract infections. Am. J. Infect. Control. 42, S236–S241 (2014).
Tambyah, P. A. & Maki, D. G. Catheter-associated urinary tract infection is rarely symptomatic: a prospective study of 1497 catheterized patients. Arch. Intern. Med. 160, 678–682 (2000).
Blodgett, T. J., Gardner, S. E., Blodgett, N. P., Peterson, L. V. & Pietraszak, M. A tool to assess the signs and symptoms of catheter-associated urinary tract infection: development and reliability. Clin. Nurs. Res. 24, 341–356 (2015).
Garimella, V. & Cellini, C. Postoperative pain control. Clin. Colon. Rectal Surg. 26, 191–196 (2013).
Dinh, A. et al. Urinary tract infections in patients with neurogenic bladder. Med. Mal. Infect. 49, 495–504 (2019).
Timm, M. R., Russell, S. K. & Hultgren, S. J. Urinary tract infections: pathogenesis, host susceptibility and emerging therapeutics. Nat. Rev. Microbiol. 23, 72–86 (2025).
Storme, O., Tiran Saucedo, J., Garcia-Mora, A., Dehesa-Davila, M. & Naber, K. G. Risk factors and predisposing conditions for urinary tract infection. Ther. Adv. Urol. 11, 1756287218814382 (2019).
Mwansa, C. M. L., Babiker, A., Satola, S., Logan, L. K. & Nadimpalli, M. L. Associations between neighbourhood-level median household income and outpatients’ risk of antibiotic non-susceptible uropathogens in a major urban centre, USA. JAC Antimicrob. Resist. 6, dlae179 (2024).
Fromer, D. L. et al. Risk factors for empiric treatment failure in US female outpatients with uncomplicated urinary tract infection: an observational study. J. Gen. Intern. Med. 40, 862–870 (2024).
Bono, M. J., Leslie, S. W. & Reygaert, W. C. Uncomplicated Urinary Tract Infections. StatPearls [Internet] https://www.ncbi.nlm.nih.gov/books/NBK470195/ (updated 21 February 2025).
Sen, A., Kaul, A. & Kaul, R. Estrogen receptors in human bladder cells regulate innate cytokine responses to differentially modulate uropathogenic E. coli colonization. Immunobiology 226, 152020 (2021).
Ackerson, B. K. et al. Risk factors for recurrent urinary tract infections among women in a large integrated health care organization in the United States. J. Infect. Dis. 230, e1101–e1111 (2024).
Anger, J. et al. Recurrent uncomplicated urinary tract infections in women: AUA/CUA/SUFU guideline. J. Urol. 202, 282–289 (2019).
Meddings, J. et al. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual. Saf. 23, 277–289 (2014).
Raz, R., Schiller, D. & Nicolle, L. E. Chronic indwelling catheter replacement before antimicrobial therapy for symptomatic urinary tract infection. J. Urol. 164, 1254–1258 (2000).
Babich, T. et al. Replacement of urinary catheter for urinary tract infections: a prospective observational study. J. Am. Geriatr. Soc. 66, 1779–1784 (2018).
Ausen, K., Fossmark, R., Spigset, O. & Pleym, H. Safety and efficacy of local tranexamic acid for the prevention of surgical bleeding in soft-tissue surgery: a review of the literature and recommendations for plastic surgery. Plast. Reconstr. Surg. 149, 774–787 (2022).
Perrin, K. et al. Catheter-associated urinary tract infection (CAUTI) in the NeuroICU: identification of risk factors and time-to-CAUTI using a case-control design. Neurocrit Care 34, 271–278 (2021).
Chen, Y. et al. Income-related inequalities in diagnosed diabetes prevalence among US adults, 2001–2018. PLoS ONE 18, e0283450 (2023).
Chen, J., Khazanchi, R., Bearman, G. & Marcelin, J. R. Racial/ethnic inequities in healthcare-associated infections under the shadow of structural racism: narrative review and call to action. Curr. Infect. Dis. Rep. 23, 17 (2021).
Li, X. et al. Effects of hyperglycemia and diabetes mellitus on coagulation and hemostasis. J. Clin. Med. 10, 2419 (2021).
Bryk-Wiazania, A. H. & Undas, A. Hypofibrinolysis in type 2 diabetes and its clinical implications: from mechanisms to pharmacological modulation. Cardiovasc. Diabetol. 20, 191 (2021).
Cronkleton, E. What Medication Can Treat a Urinary Tract Infection (UTI)? Healthline https://www.healthline.com/health/medicine-for-urinary-tract-infection#oral-antibiotics (2023).
Dunne, M. W. et al. Impact of empirical antibiotic therapy on outcomes of outpatient urinary tract infection due to nonsusceptible Enterobacterales. Microbiol. Spectr. 10, e02359–02321 (2022).
Aggarwal, N. & Leslie, S. W. Recurrent Urinary Tract Infections. StatPearls [Internet] https://www.ncbi.nlm.nih.gov/books/NBK557479/ (updated 20 January 2025).
Lee, H. S. & Le, J. in Infectious Diseases (eds Huang, V. et al.) 7–28 (PSAP, 2018).
Heimann, D., Kohnhäuser, D., Kohnhäuser, A. J. & Brönstrup, M. Antibacterials with novel chemical scaffolds in clinical development. Drugs 85, 293–323 (2025).
Sabih, A. & Leslie, S. W. Complicated Urinary Tract Infections. StatPearls [Internet] https://www.ncbi.nlm.nih.gov/books/NBK436013/ (updated 7 December 2024).
Tingsgard, S., Bastrup Israelsen, S., Jorgensen, H. L., Ostergaard, C. & Benfield, T. Early switch from intravenous to oral antibiotics for patients with uncomplicated gram-negative bacteremia. JAMA Netw. Open 7, e2352314 (2024).
Turjeman, A. et al. Duration of antibiotic treatment for gram-negative bacteremia — systematic review and individual participant data (IPD) meta-analysis. EClinicalMedicine 55, 101750 (2023).
Hollenbeck, B. L. & Rice, L. B. Intrinsic and acquired resistance mechanisms in enterococcus. Virulence 3, 421–433 (2012).
Kim, P. et al. Safety, pharmacokinetics, and pharmacodynamics of LBP-EC01, a CRISPR-Cas3-enhanced bacteriophage cocktail, in uncomplicated urinary tract infections due to Escherichia coli (ELIMINATE): the randomised, open-label, first part of a two-part phase 2 trial. Lancet Infect. Dis. 24, 1319–1332 (2024).
Kanti, S. P. Y., Csoka, I., Jojart-Laczkovich, O. & Adalbert, L. Recent advances in antimicrobial coatings and material modification strategies for preventing urinary catheter-associated complications. Biomedicines 10, 2580 (2022).
Al-Qahtani, M., Safan, A., Jassim, G. & Abadla, S. Efficacy of anti-microbial catheters in preventing catheter associated urinary tract infections in hospitalized patients: a review on recent updates. J. Infect. Public. Health 12, 760–766 (2019).
Chadha, J., Thakur, N., Chhibber, S. & Harjai, K. A comprehensive status update on modification of Foley catheter to combat catheter-associated urinary tract infections and microbial biofilms. Crit. Rev. Microbiol. 50, 168–195 (2024).
Schug, S. A. The role of COX-2 inhibitors in the treatment of postoperative pain. J. Cardiovasc. Pharmacol. 47, S82–S86 (2006).
Maki, K. C. et al. Consumption of a cranberry juice beverage lowered the number of clinical urinary tract infection episodes in women with a recent history of urinary tract infection. Am. J. Clin. Nutr. 103, 1434–1442 (2016).
Babar, A. et al. High dose versus low dose standardized cranberry proanthocyanidin extract for the prevention of recurrent urinary tract infection in healthy women: a double-blind randomized controlled trial. BMC Urol. 21, 44 (2021).
Cooper, T. E. et al. D‐mannose for preventing and treating urinary tract infections. Cochrane Database Syst. Rev. 8, CD013608 (2022).
Hayward, G. et al. d-Mannose for prevention of recurrent urinary tract infection among women: a randomized clinical trial. JAMA Intern. Med. 184, 619–628 (2024).
La Bella, A. A., Molesan, A., Wollin, D. A., Paul, S. & Flores-Mireles, A. L. Initial antimicrobial testing of a novel reusable intermittent urinary catheter system and catheter reprocessing device. Urology 193, 8–15 (2024).
Etefia, E. in Escherichia coli — Old and New Insights (ed. Erjavec, M. S.) (IntechOpen, 2021).
Carere-Sigl, A., Nowakowska, J., Hevey, R., Khanna, N. & Ernst, B. in Carbohydrate Chemistry: Chemical and Biological Approaches, Volume 45 (eds Rauter, A. P., Lindhorst, T. K., Queneau. Y. (Royal Society of Chemistry, 2021).
Spaulding, C. N. & Hultgren, S. J. Adhesive pili in UTI pathogenesis and drug development. Pathogens 5, 30 (2016).
Chagneau, C. V. et al. HlyF, an underestimated virulence factor of uropathogenic Escherichia coli. Clin. Microbiol. Infect. 29, 1449.e1441–1449.e1449 (2023).
Tanaka, R. et al. Cyclic-di-AMP confers an invasive phenotype on Escherichia coli through elongation of flagellin filaments. Gut Pathog. 16, 6 (2024).
Lopez-Banda, D. A. et al. Identification of virulence factors genes in Escherichia coli isolates from women with urinary tract infection in Mexico. Biomed. Res. Int. 2014, 959206 (2014).
Bunduki, G. K. et al. Virulence factors and antimicrobial resistance of uropathogenic Escherichia coli (UPEC) isolated from urinary tract infections: a systematic review and meta-analysis. BMC Infect. Dis. 21, 753 (2021).
Zou, Z. et al. E. coli catheter-associated urinary tract infections are associated with distinctive virulence and biofilm gene determinants. JCI Insight 8, e161461 (2023).
Mohamed, E. H. et al. Study of biofilm, virulence genes, and risk factors in urinary catheter-associated Escherichia coli infections. Jundishapur J. Microbiol. 17, 1–9 (2024).
Lopatto, E. D. B. et al. Conformational ensembles in Klebsiella pneumoniae FimH impact uropathogenesis. Proc. Natl Acad. Sci. USA 121, e2409655121 (2024).
Clegg, S. & Murphy, C. N. in Urinary Tract Infections: Molecular Pathogenesis and Clinical Management (eds. Mulvey, M. A. et al.) 435-457 (Oxford Univ. Press, 2017).
Govindarajan, D. K. & Kandaswamy, K. Virulence factors of uropathogens and their role in host pathogen interactions. Cell Surf. 8, 100075 (2022).
Schaffer, J. N. & Pearson, M. M. Proteus mirabilis and urinary tract infections. Microbiol. Spectr. https://doi.org/10.1128/microbiolspec.UTI-0017-2013 (2015).
Wasfi, R., Hamed, S. M., Amer, M. A. & Fahmy, L. I. Proteus mirabilis biofilm: development and therapeutic strategies. Front. Cell Infect. Microbiol. 10, 414 (2020).
Newman, J. W., Floyd, R. V. & Fothergill, J. L. The contribution of Pseudomonas aeruginosa virulence factors and host factors in the establishment of urinary tract infections. FEMS Microbiol. Lett. 364, fnx124 (2017).
Romling, U., Galperin, M. Y. & Gomelsky, M. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. Rev. 77, 1–52 (2013).
Cole, S. J., Records, A. R., Orr, M. W., Linden, S. B. & Lee, V. T. Catheter-associated urinary tract infection by Pseudomonas aeruginosa is mediated by exopolysaccharide-independent biofilms. Infect. Immun. 82, 2048–2058 (2014).
Jackson-Litteken, C. D. et al. InvL, an invasin-like adhesin, is a type II secretion system substrate required for Acinetobacter baumannii uropathogenesis. mBio 13, e0025822 (2022).
Kundra, S. et al. c-di-AMP is essential for the virulence of Enterococcus faecalis. Infect. Immun. 89, e0036521 (2021).
Colomer-Winter, C. et al. Manganese acquisition is essential for virulence of Enterococcus faecalis. PLoS Pathog. 14, e1007102 (2018).
Lam, L. N., Brunson, D. N., Molina, J. J., Flores-Mireles, A. L. & Lemos, J. A. The AdcACB/AdcAII system is essential for zinc homeostasis and an important contributor of Enterococcus faecalis virulence. Virulence 13, 592–608 (2022).
Xu, K. et al. Staphylococcus aureus ST1 promotes persistent urinary tract infection by highly expressing the urease. Front. Microbiol. 14, 1101754 (2023).
Paudel, S. et al. Defining the roles of pyruvate oxidation, TCA cycle, and mannitol metabolism in methicillin-resistant Staphylococcus aureus catheter-associated urinary tract infection. Microbiol. Spectr. 11, e05365–e05322 (2023).
Ismail, M. G., El-Haliem, A., Farouk, N. & Aboelmagd, E. K. Assessment of virulence factors and antifungal susceptibility of Candida species isolated from catheter associated urinary tract infections. Al-Azhar Int. Med. J. 1, 179–188 (2020).
Letica-Kriegel, A. S. et al. Identifying the risk factors for catheter-associated urinary tract infections: a large cross-sectional study of six hospitals. BMJ Open 9, e022137 (2019).
Shankar, M., Narasimhappa, S. & Madhura, N. S. Urinary tract infection in chronic kidney disease population: a clinical observational study. Cureus 13, e12486 (2021).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks Florian Wagenlehner, Sharyl Justice and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Molina, J.J., Flores-Mireles, A.L. CAUTIon — not all UTIs are the same. Nat Rev Urol 22, 799–814 (2025). https://doi.org/10.1038/s41585-025-01065-z
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41585-025-01065-z
This article is cited by
-
Multifactor machine learning models for predicting urinary tract infections: a pilot study
International Urology and Nephrology (2025)