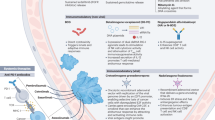
Abstract
Low-grade non-muscle invasive bladder cancer is a specific category of bladder cancer with a favourable prognosis; however, its management presents several challenges. The risk of stage progression is very low, but approximately half of patients will experience recurrence within the first 5 years after diagnosis. This high propensity for recurrence, coupled with the threat of progression, mandates ongoing surveillance. However, the optimal frequency and duration of follow-up monitoring remain undefined. Current management strategies for low-grade non-muscle invasive bladder cancer rely heavily on routine office cystoscopy, with few advances in diagnostic and treatment options over the past 25 years. Our basic understanding of disease biology has substantially advanced. However, at present, considerable variations in clinical practice exist, with implications for increased financial and treatment burden for patients and health care systems. Molecular signatures and biomarker discoveries are crucial to understand disease behaviour and inform novel treatment strategies. Emerging therapies, such as advanced drug-delivery systems, immunomodulatory agents and targeted therapies, offer the potential to improve patient outcomes, streamline management and reduce the need for surveillance cystoscopies. Actionable avenues for future research in the field include prospective validation of novel biomarkers and therapies with the ultimate aim of optimizing patient care and reducing health care costs.
Key points
-
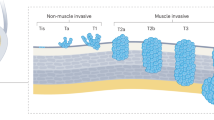
Tumour grade is one of the most important prognostic features in non-muscle invasive bladder cancer (NMIBC) and low-grade disease accounts for half of patients with NMIBC at presentation. Low-grade tumours are characterized by a high propensity to recur, with a lifetime average of 6.6 recurrences per patient. The risk of recurrence is highest within the first 5 years following diagnosis and reduces thereafter. This feature of disease contributes to the overall high cost of bladder cancer care.
-
Low-grade NMIBC is associated with a low rate of progression to an increased stage or grade (3–19%). Risk of progression to muscle-invasive bladder cancer is 1.6% overall but can be as high as 8.3% in patients who have multiple risk factors as defined by the International Bladder Cancer Group intermediate-risk NMIBC scoring system.
-
Current management challenges in low-grade NMIBC reflect the lack of consensus of the most optimal surveillance frequency and intensity. High use of surveillance cystoscopy results in escalating health care costs without affecting disease outcome.
-
Low-grade NMIBC exhibits relative molecular homogeneity and low tumour mutational burden compared with high-grade NMIBC and MIBC, with increased prevalence of gain-of-function alterations in FGFR3, RAS and PIK3CA. Low-grade NMIBC lacks molecular aberrations in commonly mutated genes prevalent in advanced disease (TP53, CDKN1A, RB1, ERCC2, ERBB3 and FBXW7). These differences highlight distinct pathways of oncogenesis and hint at differences in therapeutic strategies.
-
Bladder cancer is unique in that tumour cells and associated proteins are continuously in contact with and shed into the urine, which can be leveraged using non-invasive urine-based biomarkers. Urine cytology remains one of the only biomarkers endorsed by professional guidelines in NMIBC surveillance algorithms, but has low sensitivity, especially for low-grade disease.
-
Emerging therapies and innovations in drug delivery, immunomodulation and targeted treatments offer promising avenues to enhance the efficacy of treatment while potentially reducing the need for invasive follow-up procedures.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Zhang, Y. et al. The global landscape of bladder cancer incidence and mortality in 2020 and projections to 2040. J. Glob. Health 13, 04109 (2023).
Jubber, I. et al. Epidemiology of bladder cancer in 2023: a systematic review of risk factors. Eur. Urol. 84, 176–190 (2023).
Lopez-Beltran, A., Cookson, M. S., Guercio, B. J. & Cheng, L. Advances in diagnosis and treatment of bladder cancer. BMJ 384, e076743 (2024).
van Rhijn, B. W. G. et al. Prognostic value of the WHO1973 and WHO2004/2016 classification systems for grade in primary Ta/T1 non-muscle-invasive bladder cancer: a multicenter European Association of Urology non-muscle-invasive bladder cancer guidelines panel study. Eur. Urol. Oncol. 4, 182–191 (2021).
Falke, J. & Witjes, J. A. Contemporary management of low-risk bladder cancer. Nat. Rev. Urol. 8, 42–49 (2011).
Messing, E. M. et al. Effect of intravesical instillation of gemcitabine vs saline immediately following resection of suspected low-grade non-muscle-invasive bladder cancer on tumor recurrence: SWOG S0337 randomized clinical trial. JAMA 319, 1880–1888 (2018).
Joyce, D. D., Sharma, V. & Williams, S. B. Cost-effectiveness and economic impact of bladder cancer management: an updated review of the literature. Pharmacoeconomics 41, 751–769 (2023).
Holzbeierlein, J. M. et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline: 2024 amendment. J. Urol. 211, 533–538 (2024).
Sylvester, R. J. et al. European Association of Urology (EAU) prognostic factor risk groups for non-muscle-invasive bladder cancer (NMIBC) incorporating the WHO 2004/2016 and WHO 1973 classification systems for grade: an update from the EAU NMIBC guidelines panel. Eur. Urol. 79, 480–488 (2021).
Bree, K. K. et al. Management, surveillance patterns, and costs associated with low-grade papillary stage Ta non-muscle-invasive bladder cancer among older adults, 2004–2013. JAMA Netw. Open. 5, e223050–e223050 (2022).
Jung, A. et al. Quality of life in non-muscle-invasive bladder cancer survivors: a systematic review. Cancer Nurs. 42, E21–E33 (2019).
Koo, K. et al. The burden of cystoscopic bladder cancer surveillance: anxiety, discomfort, and patient preferences for decision making. Urology 108, 122–128 (2017).
Chang, S. S. et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J. Urol. 196, 1021–1029 (2016).
Babjuk, M. et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur. Urol. 71, 447–461 (2017).
Gontero, P. et al. European Association of Urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ) — a summary of the 2024 guidelines update. Eur. Urol. 86, 531–549 (2024).
Raspollini, M. R. et al. News in the classification of WHO 2022 bladder tumors. Pathologica 115, 32–40 (2022).
Barkan, G. A. et al. The Paris system for reporting urinary cytology: the quest to develop a standardized terminology. Acta Cytol. 60, 185–197 (2016).
Humphrey, P. A. Tumor amount in prostatic tissues in relation to patient outcome and management. Am. J. Clin. Pathol. 131, 7–10 (2009).
Reis, L. O. et al. Significance of a minor high-grade component in a low-grade noninvasive papillary urothelial carcinoma of bladder. Hum. Pathol. 47, 20–25 (2016).
US National Library of Medicine. ClinicalTrials.gov https://Clinicaltrials.Gov/Study/NCT06319820 (2025).
Matulay, J. T. et al. Risk-adapted management of low-grade bladder tumours: recommendations from the International Bladder Cancer Group (IBCG). BJU Int. 125, 497–505 (2020).
Sylvester, R. J. et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur. Urol. 49, 466–465 (2006). discussion 475–477.
Soria, F. et al. Clinical validation of the intermediate-risk non-muscle-invasive bladder cancer scoring system and substratification model proposed by the International Bladder Cancer Group: a multicenter young academic urologists urothelial working group collaboration. Eur. Urol. Oncol. 7, 1497–1503 (2024).
Ma, J. et al. Long-term recurrence rates of low-risk non-muscle-invasive bladder cancer-how long is cystoscopic surveillance necessary? Eur. Urol. Focus. 10, 189–196 (2024).
Miyamoto, H. et al. Low-grade papillary urothelial carcinoma of the urinary bladder: a clinicopathologic analysis of a post-World Health Organization/International Society of Urological Pathology classification cohort from a single academic center. Arch. Pathol. Lab. Med. 134, 1160–1163 (2010).
Herr, H. W., Donat, S. M. & Reuter, V. E. Management of low grade papillary bladder tumors. J. Urol. 178, 1201–1205 (2007). discussion 1205.
Fitzpatrick, J. M., West, A. B., Butler, M. R., Lane, V. & O’Flynn, J. D. Superficial bladder tumors (stage pTa, grades 1 and 2): the importance of recurrence pattern following initial resection. J. Urol. 135, 920–922 (1986).
Hernández, V. et al. Long-term oncological outcomes of an active surveillance program in recurrent low grade Ta bladder cancer. Urol. Oncol. 34, 165.e19–23 (2016).
Zhang, J. H. et al. National complication and cost burden of transurethral resection of bladder tumor for bladder cancer. Urol. Oncol. 43, 469.e1–469.e11 (2025).
Dyrskjøt, L. et al. Bladder cancer. Nat. Rev. Dis. Prim. 9, 58 (2023).
Van Batavia, J. et al. Bladder cancers arise from distinct urothelial sub-populations. Nat. Cell Biol. 16, 982–991 (2014). 1–5.
Acharya, P. et al. Distribution of the tight junction proteins ZO-1, occludin, and claudin-4, -8, and -12 in bladder epithelium. Am. J. Physiol. Renal Physiol. 287, F305–F318 (2004).
Wiessner, G. B., Plumber, S. A., Xiang, T. & Mendelsohn, C. L. Development, regeneration and tumorigenesis of the urothelium. Dev. Camb. Engl. 149, dev198184 (2022).
Shin, K. et al. Cellular origin of bladder neoplasia and tissue dynamics of its progression to invasive carcinoma. Nat. Cell Biol. 16, 469–478 (2014).
Papafotiou, G. et al. KRT14 marks a subpopulation of bladder basal cells with pivotal role in regeneration and tumorigenesis. Nat. Commun. 7, 11914 (2016).
Li, R. et al. Effects of thiazolidinedione in patients with active bladder cancer. BJU Int. 121, 244–251 (2018).
Warrick, J. I. et al. FOXA1, GATA3 and PPARɣ cooperate to drive luminal subtype in bladder cancer: a molecular analysis of established human cell lines. Sci. Rep. 6, 38531 (2016).
Hedegaard, J. et al. Comprehensive transcriptional analysis of early-stage urothelial carcinoma. Cancer Cell 30, 27–42 (2016).
Tate, T. et al. Pparg signaling controls bladder cancer subtype and immune exclusion. Nat. Commun. 12, 6160 (2021).
Halstead, A. M. et al. Bladder-cancer-associated mutations in RXRA activate peroxisome proliferator-activated receptors to drive urothelial proliferation. eLife 6, e30862 (2017).
Hurst, C. D. et al. Genomic subtypes of non-invasive bladder cancer with distinct metabolic profile and female gender bias in KDM6A mutation frequency. Cancer Cell 32, 701–715.e7 (2017).
Erdmann, E., Harding, S., Lam, H. & Perez, A. Ten-year observational follow-up of PROactive: a randomized cardiovascular outcomes trial evaluating pioglitazone in type 2 diabetes. Diabetes Obes. Metab. 18, 266–273 (2016).
Lewis, J. D. et al. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. JAMA 314, 265–277 (2015).
Tate, T. et al. Combined Mek inhibition and Pparg activation eradicates muscle invasive bladder cancer in a mouse model of BBN-induced carcinogenesis. BioRxiv Prepr. Serv. Biol. https://doi.org/10.1101/2023.08.19.553961 (2023).
Sjödahl, G. et al. A molecular taxonomy for urothelial carcinoma. Clin. Cancer Res. 18, 3377–3386 (2012).
Guo, Y. et al. TSC1 involvement in bladder cancer: diverse effects and therapeutic implications. J. Pathol. 230, 17–27 (2013).
Hurst, C. D. & Knowles, M. A. Mutational landscape of non-muscle-invasive bladder cancer. Urol. Oncol. 40, 295–303 (2022).
Minner, S. et al. Y chromosome loss is a frequent early event in urothelial bladder cancer. Pathology 42, 356–359 (2010).
Pietzak, E. J. et al. Next-generation sequencing of nonmuscle invasive bladder cancer reveals potential biomarkers and rational therapeutic targets. Eur. Urol. 72, 952–959 (2017).
Ascione, C. M. et al. Role of FGFR3 in bladder cancer: treatment landscape and future challenges. Cancer Treat. Rev. 115, 102530 (2023).
Wu, Y.-M. et al. Identification of targetable FGFR gene fusions in diverse cancers. Cancer Discov. 3, 636–647 (2013).
Wang, C. G., Peiris, M. N., Meyer, A. N., Nelson, K. N. & Donoghue, D. J. Oncogenic driver FGFR3-TACC3 requires five coiled-coil heptads for activation and disulfide bond formation for stability. Oncotarget 14, 133–145 (2023).
di Martino, E., L’Hôte, C. G., Kennedy, W., Tomlinson, D. C. & Knowles, M. A. Mutant fibroblast growth factor receptor 3 induces intracellular signaling and cellular transformation in a cell type- and mutation-specific manner. Oncogene 28, 4306–4316 (2009).
Tomlinson, D. C., Baldo, O., Harnden, P. & Knowles, M. A. FGFR3 protein expression and its relationship to mutation status and prognostic variables in bladder cancer. J. Pathol. 213, 91–98 (2007).
Hernández, S. et al. Prospective study of FGFR3 mutations as a prognostic factor in nonmuscle invasive urothelial bladder carcinomas. J. Clin. Oncol. 24, 3664–3671 (2006).
van Rhijn, B. W. et al. The fibroblast growth factor receptor 3 (FGFR3) mutation is a strong indicator of superficial bladder cancer with low recurrence rate. Cancer Res. 61, 1265–1268 (2001).
Jebar, A. H. et al. FGFR3 and Ras gene mutations are mutually exclusive genetic events in urothelial cell carcinoma. Oncogene 24, 5218–5225 (2005).
Zhang, Z. T. et al. Role of Ha-ras activation in superficial papillary pathway of urothelial tumor formation. Oncogene 20, 1973–1980 (2001).
Mo, L. et al. Hyperactivation of Ha-ras oncogene, but not Ink4a/Arf deficiency, triggers bladder tumorigenesis. J. Clin. Invest. 117, 314–325 (2007).
Urakami, S. et al. Recurrent transitional cell carcinoma in a child with the Costello syndrome. J. Urol. 168, 1133–1134 (2002).
Hurst, C. D. et al. Stage-stratified molecular profiling of non-muscle-invasive bladder cancer enhances biological, clinical, and therapeutic insight. Cell Rep. Med. 2, 100472 (2021).
López-Knowles, E. et al. PIK3CA mutations are an early genetic alteration associated with FGFR3 mutations in superficial papillary bladder tumors. Cancer Res. 66, 7401–7404 (2006).
Facchinetti, F. et al. Resistance to selective FGFR inhibitors in FGFR-driven urothelial cancer. Cancer Discov. 13, 1998–2011 (2023).
Wolff, E. M. et al. Unique DNA methylation patterns distinguish noninvasive and invasive urothelial cancers and establish an epigenetic field defect in premalignant tissue. Cancer Res. 70, 8169–8178 (2010).
Hu, D. et al. The MLL3/MLL4 branches of the COMPASS family function as major histone H3K4 monomethylases at enhancers. Mol. Cell. Biol. 33, 4745–4754 (2013).
Yoo, K. H. et al. Histone demethylase KDM6A controls the mammary luminal lineage through enzyme-independent mechanisms. Mol. Cell. Biol. 36, 2108–2120 (2016).
Barrows, D., Feng, L., Carroll, T. S. & Allis, C. D. Loss of UTX/KDM6A and the activation of FGFR3 converge to regulate differentiation gene-expression programs in bladder cancer. Proc. Natl Acad. Sci. USA 117, 25732–25741 (2020).
Babjuk, M. et al. European Association of Urology guidelines on non-muscle-invasive bladder cancer (Ta, T1, and carcinoma in situ). Eur. Urol. 81, 75–94 (2022).
Papanicolaou, G. N. Cytology of the urine sediment in neoplasms of the urinary tract. J. Urol. 57, 375–379 (1947).
Raab, S. S., Grzybicki, D. M., Vrbin, C. M. & Geisinger, K. R. Urine cytology discrepancies: frequency, causes, and outcomes. Am. J. Clin. Pathol. 127, 946–953 (2007).
Nguyen, G.-K. & Smith, R. Repair renal tubular cells: a potential false-positive diagnosis in urine cytology. Diagn. Cytopathol. 31, 342–346 (2004).
Brown, F. M. Urine cytology. It is still the gold standard for screening? Urol. Clin. North Am. 27, 25–37 (2000).
Choi, S.-Y., Kim, K.-H., Suh, K.-S. & Yeo, M.-K. Diagnostic significance of dual immunocytochemical staining of p53/cytokeratin20 on liquid-based urine cytology to detect urothelial carcinoma. Cytojournal 17, 3 (2020).
Yafi, F. A. et al. Prospective analysis of sensitivity and specificity of urinary cytology and other urinary biomarkers for bladder cancer. Urol. Oncol. 33, 66.e25–66.e31 (2015).
Matulay, J. T. et al. Variability in adherence to guidelines based management of nonmuscle invasive bladder cancer among Society of Urologic Oncology (SUO) members. Urol. Oncol. 38, 796.e1–796.e6 (2020).
Allard, P., Fradet, Y., Têtu, B. & Bernard, P. Tumor-associated antigens as prognostic factors for recurrence in 382 patients with primary transitional cell carcinoma of the bladder. Clin. Cancer Res. 1, 1195–1202 (1995).
Comploj, E. et al. uCyt+/ImmunoCyt and cytology in the detection of urothelial carcinoma. Cancer Cytopathol. 121, 392–397 (2013).
Hirasawa, Y. et al. Diagnostic performance of OncuriaTM, a urinalysis test for bladder cancer. J. Transl. Med. 19, 141–141 (2021).
Wang, Z. et al. Evaluation of the NMP22 BladderChek test for detecting bladder cancer: a systematic review and meta-analysis. Oncotarget 8, 100648–100656 (2017).
Landman, J., Chang, Y., Kavaler, E., Droller, M. J. & Liu, B. C.-S. Sensitivity and specificity of NMP-22, telomerase, and BTA in the detection of human bladder cancer. Urology 52, 398–402 (1998).
Sarosdy, M. F. et al. Improved detection of recurrent bladder cancer using the Bard BTA stat Test. Urology 50, 349–353 (1997).
Thomas, L. et al. Multicenter trial of the quantitative BTA TRAK assay in the detection of bladder cancer. Clin. Chem. 45, 472–477 (1999).
Darling, D., Luxmanan, C., O’Sullivan, P., Lough, T. & Suttie, J. Clinical utility of Cxbladder for the diagnosis of urothelial carcinoma. Adv. Ther. 34, 1087–1096 (2017).
Laukhtina, E. et al. Diagnostic accuracy of novel urinary biomarker tests in non-muscle-invasive bladder cancer: a systematic review and network meta-analysis. Eur. Urol. Oncol. 4, 927–942 (2021).
Kavalieris, L. et al. Performance characteristics of a multigene urine biomarker test for monitoring for recurrent urothelial carcinoma in a multicenter study. J. Urol. 197, 1419–1426 (2017).
Cancel-Tassin, G. et al. Assessment of Xpert bladder cancer monitor test performance for the detection of recurrence during non-muscle invasive bladder cancer follow-up. World J. Urol. 39, 3329–3335 (2021).
Sharma, G., Sharma, A., Krishna, M., Devana, S. K. & Singh, S. K. Xpert bladder cancer monitor in surveillance of bladder cancer: systematic review and meta-analysis. Urol. Oncol. Semin. Orig. Investig. 40, 163.e1–163.e9 (2022).
Witjes, J. A. et al. Performance of the bladder EpiCheckTM methylation test for patients under surveillance for non-muscle-invasive bladder cancer: results of a multicenter, prospective, blinded clinical trial. Eur. Urol. Oncol. 1, 307–313 (2018).
Beukers, W. et al. FGFR3, TERT and OTX1 as a urinary biomarker combination for surveillance of patients with bladder cancer in a large prospective multicenter study. J. Urol. 197, 1410–1418 (2017).
Moonen, P. M. J. et al. UroVysion compared with cytology and quantitative cytology in the surveillance of non-muscle-invasive bladder cancer. Eur. Urol. 51, 1275–1280 (2007).
Lotan, Y. et al. Evaluation of the fluorescence in situ hybridization test to predict recurrence and/or progression of disease after bacillus Calmette-Guérin for primary high grade nonmuscle invasive bladder cancer: results from a prospective multicenter trial. J. Urol. 202, 920–926 (2019).
HALLING, K. C. et al. A comparison of cytology and fluorescence in situ hybridization for the detection of urothelial carcinoma. J. Urol. 164, 1768–1775 (2000).
Dudley, J. C. et al. Detection and surveillance of bladder cancer using urine tumor DNA. Cancer Discov. 9, 500–509 (2019).
Salari, K. et al. Development and multicenter case-control validation of urinary comprehensive genomic profiling for urothelial carcinoma diagnosis, surveillance, and risk-prediction. Clin. Cancer Res. 29, 3668–3680 (2023).
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 507, 315–322 (2014).
Prip, F. et al. Comprehensive genomic characterization of early-stage bladder cancer. Nat. Genet. 57, 115–125 (2025).
Huelster, H. L. et al. Novel use of circulating tumor DNA to identify muscle-invasive and non-organ-confined upper tract urothelial carcinoma. Eur. Urol. 85, 283–292 (2024).
Chauhan, P. S. et al. Urine tumor DNA detection of minimal residual disease in muscle-invasive bladder cancer treated with curative-intent radical cystectomy: a cohort study. PLoS Med. 18, e1003732 (2021).
Shkolyar, E. et al. Augmented bladder tumor detection using deep learning. Eur. Urol. 76, 714–718 (2019).
Compérat, E. M. et al. Grading of urothelial carcinoma and the new ‘World Health Organisation classification of tumours of the urinary system and male genital organs 2016’. Eur. Urol. Focus. 5, 457–466 (2019).
Jansen, I. et al. Automated detection and grading of non-muscle-invasive urothelial cell carcinoma of the bladder. Am. J. Pathol. 190, 1483–1490 (2020).
Rose, K. et al. Complimentary genomic, pathologic, and artificial intelligence analysis on low-grade noninvasive bladder cancer to predict downstream recurrence. J. Clin. Oncol. 41, 6 (2023).
US National Library of Medicine. ClinicalTrials.gov https://Clinicaltrials.Gov/Study/NCT03988309 (2025).
Chou, R. et al. Intravesical therapy for the treatment of nonmuscle invasive bladder cancer: a systematic review and meta-analysis. J. Urol. 197, 1189–1199 (2017).
Dalton, J. T., Wientjes, M. G., Badalament, R. A., Drago, J. R. & Au, J. L. Pharmacokinetics of intravesical mitomycin C in superficial bladder cancer patients. Cancer Res. 51, 5144–5152 (1991).
Addeo, R. et al. Randomized phase III trial on gemcitabine versus mytomicin in recurrent superficial bladder cancer: evaluation of efficacy and tolerance. J. Clin. Oncol. 28, 543–548 (2010).
Sylvester, R. J., Oosterlinck, W. & van der Meijden, A. P. M. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. J. Urol. 171, 2186–2190 (2004). quiz 2435.
Sylvester, R. J. et al. Systematic review and individual patient data meta-analysis of randomized trials comparing a single immediate instillation of chemotherapy after transurethral resection with transurethral resection alone in patients with stage pTa-pT1 urothelial carcinoma of the bladder: which patients benefit from the instillation? Eur. Urol. 69, 231–244 (2016).
Gudjónsson, S. et al. Should all patients with non-muscle-invasive bladder cancer receive early intravesical chemotherapy after transurethral resection? The results of a prospective randomised multicentre study. Eur. Urol. 55, 773–780 (2009).
Bosschieter, J. et al. Value of an immediate intravesical instillation of mitomycin c in patients with non-muscle-invasive bladder cancer: a prospective multicentre randomised study in 2243 patients. Eur. Urol. 73, 226–232 (2018).
Tan, W. S. et al. Intermediate-risk non-muscle-invasive bladder cancer: updated consensus definition and management recommendations from the International Bladder Cancer Group. Eur. Urol. Oncol. 5, 505–516 (2022).
Oddens, J. et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guérin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur. Urol. 63, 462–472 (2013).
Böhle, A., Jocham, D. & Bock, P. R. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J. Urol. 169, 90–95 (2003).
Chen, S., Zhang, N., Shao, J. & Wang, X. Maintenance versus non-maintenance intravesical Bacillus Calmette-Guerin instillation for non-muscle invasive bladder cancer: a systematic review and meta-analysis of randomized clinical trials. Int. J. Surg. 52, 248–257 (2018).
Hinotsu, S. et al. Maintenance therapy with bacillus Calmette-Guérin Connaught strain clearly prolongs recurrence-free survival following transurethral resection of bladder tumour for non-muscle-invasive bladder cancer. BJU Int. 108, 187–195 (2011).
Malmström, P.-U. et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non-muscle-invasive bladder cancer. Eur. Urol. 56, 247–256 (2009).
Marttila, T. et al. Intravesical Bacillus Calmette-Guérin versus combination of epirubicin and interferon-α2a in reducing recurrence of non-muscle-invasive bladder carcinoma: FinnBladder-6 study. Eur. Urol. 70, 341–347 (2016).
Ojea, A. et al. A multicentre, randomised prospective trial comparing three intravesical adjuvant therapies for intermediate-risk superficial bladder cancer: low-dose bacillus Calmette-Guerin (27 mg) versus very low-dose bacillus Calmette-Guerin (13.5 mg) versus mitomycin C. Eur. Urol. 52, 1398–1406 (2007).
Sylvester, R. J. et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guérin, and bacillus Calmette-Guérin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur. Urol. 57, 766–773 (2010).
Au, J. L. et al. Methods to improve efficacy of intravesical mitomycin C: results of a randomized phase III trial. J. Natl Cancer Inst. 93, 597–604 (2001).
Schmidt, S. et al. Intravesical Bacillus Calmette-Guérin versus mitomycin C for Ta and T1 bladder cancer: abridged summary of the Cochrane Review. Investig. Clin. Urol. 61, 349–354 (2020).
Tan, W. S. et al. Sequential intravesical gemcitabine and docetaxel is an alternative to Bacillus Calmette-Guérin for the treatment of intermediate-risk non-muscle-invasive bladder cancer. Eur. Urol. Oncol. 6, 531–534 (2023).
Contieri, R. et al. Deintensification of treatment for low-grade bladder tumors: a collaborative review by the international bladder cancer group (IBCG). Eur. Urol. Oncol. 8, 179–189 (2025).
Soloway, M. S., Bruck, D. S. & Kim, S. S. Expectant management of small, recurrent, noninvasive papillary bladder tumors. J. Urol. 170, 438–441 (2003).
Hernández, V. et al. Safety of active surveillance program for recurrent nonmuscle-invasive bladder carcinoma. Urology 73, 1306–1310 (2009).
Contieri, R. et al. Long-term follow-up and factors associated with active surveillance failure for patients with non-muscle-invasive bladder cancer: the bladder cancer Italian active surveillance (BIAS) experience. Eur. Urol. Oncol. 5, 251–255 (2022).
Tan, W. S. et al. International Bladder Cancer Group intermediate-risk nonmuscle-invasive bladder cancer scoring system predicts outcomes of patients on active surveillance. J. Urol. 210, 763–770 (2023).
Ge, P. et al. Oncological outcome of primary and secondary muscle-invasive bladder cancer: a systematic review and meta-analysis. Sci. Rep. 8, 7543 (2018).
Gofrit, O. N., Zorn, K. C., Shikanov, S. & Steinberg, G. D. Marker lesion experiments in bladder cancer-what have we learned? J. Urol. 183, 1678–1684 (2010).
Gregg, J. R. et al. Short term complications from transurethral resection of bladder tumor. Can. J. Urol. 23, 8198–8203 (2016).
Gontero, P. et al. Phase II study to investigate the ablative efficacy of intravesical administration of gemcitabine in intermediate-risk superficial bladder cancer (SBC). Eur. Urol. 46, 339–343 (2004).
Colombo, R. et al. Neoadjuvant short-term intensive intravesical mitomycin C regimen compared with weekly schedule for low-grade recurrent non-muscle-invasive bladder cancer: preliminary results of a randomised phase 2 study. Eur. Urol. 62, 797–802 (2012).
Racioppi, M. et al. Chemoablation with intensive intravesical mitomycin C treatment: a new approach for non-muscle-invasive bladder cancer. Eur. Urol. Oncol. 2, 576–583 (2019).
Lindgren, M. S. et al. DaBlaCa-13 study: oncological outcome of short-term, intensive chemoresection with mitomycin in nonmuscle invasive bladder cancer: primary outcome of a randomized controlled trial. J. Clin. Oncol. 41, 206–211 (2023).
Mostafid, A. H. et al. CALIBER: a phase II randomized feasibility trial of chemoablation with mitomycin-C vs surgical management in low-risk non-muscle-invasive bladder cancer. BJU Int. 125, 817–826 (2020).
Chevli, K. K. et al. Primary chemoablation of low-grade intermediate-risk nonmuscle-invasive bladder cancer using UGN-102, a mitomycin-containing reverse thermal gel (Optima II): a phase 2b, open-label, single-arm trial. J. Urol. 207, 61–69 (2022).
Prasad, S. M. et al. Treatment of low-grade Intermediate-risk nonmuscle-invasive bladder cancer with UGN-102 ± transurethral resection of bladder tumor compared to transurethral resection of bladder tumor monotherapy: a randomized, controlled, phase 3 trial (ATLAS). J. Urol. 210, 619–629 (2023).
Prasad, S. M. et al. Primary chemoablation of recurrent low-grade intermediate-risk nonmuscle-invasive bladder cancer with UGN-102: a single-arm, open-label, phase 3 trial (ENVISION). J. Urol. 213, 205–216 (2025).
Stover, A. M. et al. Perceived impact on patient routines/responsibilities for surgery and a nonsurgical primary treatment option in recurrent low-grade intermediate-risk nonmuscle-invasive bladder cancer: findings from the ENVISION phase 3 trial. J. Urol. 214, 18–31 (2025).
Abufaraj, M., Mostafid, H., Shariat, S. F. & Babjuk, M. What to do during Bacillus Calmette–Guérin shortage? Valid strategies based on evidence. Curr. Opin. Urol. 28, 570–576 (2018).
Racioppi, M. et al. ElectroMotive drug administration (EMDA) of mitomycin C as first-line salvage therapy in high risk ‘BCG failure’ non muscle invasive bladder cancer: 3 years follow-up outcomes. BMC Cancer 18, 1224 (2018).
Arends, T. J. H. et al. Results of a randomised controlled trial comparing intravesical chemohyperthermia with mitomycin C versus Bacillus Calmette-Guérin for adjuvant treatment of patients with intermediate- and high-risk non-muscle-invasive bladder cancer. Eur. Urol. 69, 1046–1052 (2016).
Slater, S. E. et al. The effects and effectiveness of electromotive drug administration and chemohyperthermia for treating non-muscle invasive bladder cancer. Ann. R. Coll. Surg. Engl. 96, 415–419 (2014).
Zazzara, M. et al. Electromotive drug administration of mitomycin C (EMDA/MMC) versus intravesical immunotherapy with Bacillus Calmette-Guérin (BCG) in intermediate and high risk non muscle invasive bladder cancer. Urol. Int. 107, 64–71 (2023).
Liem, E. I. M. L., Crezee, H., de la Rosette, J. J. & de Reijke, T. M. Chemohyperthermia in non-muscle-invasive bladder cancer: An overview of the literature and recommendations. Int. J. Hyperthermia 32, 363–373 (2016).
van der Heijden, A. G., Verhaegh, G., Jansen, C. F. J., Schalken, J. A. & Witjes, J. A. Effect of hyperthermia on the cytotoxicity of 4 chemotherapeutic agents currently used for the treatment of transitional cell carcinoma of the bladder: an in vitro study. J. Urol. 173, 1375–1380 (2005).
Colombo, R., Salonia, A., Leib, Z., Pavone-Macaluso, M. & Engelstein, D. Long-term outcomes of a randomized controlled trial comparing thermochemotherapy with mitomycin-C alone as adjuvant treatment for non-muscle-invasive bladder cancer (NMIBC). BJU Int. 107, 912–918 (2011).
Tan, W. S. et al. Adjuvant intravesical chemohyperthermia versus passive chemotherapy in patients with intermediate-risk non-muscle-invasive bladder cancer (HIVEC-II): a phase 2, open-label, randomised controlled trial. Eur. Urol. 83, 497–504 (2023).
Zhao, H. et al. Intravesical chemohyperthermia vs. Bacillus Calmette-Guerin instillation for intermediate- and high-risk non-muscle invasive bladder cancer: a systematic review and meta-analysis. Front. Surg. 8, 775527 (2021).
Vilaseca, A. et al. First safety and efficacy results of the TAR-210 erdafitinib (erda) intravesical delivery system in patients (pts) with non-muscle-invasive bladder cancer (NMIBC) with select FGFR alterations (alt). Ann. Oncol. 34, S1343 (2023).
Tyson, M. D. et al. Safety, tolerability, and preliminary efficacy of TAR-200 in patients with muscle-invasive bladder cancer who refused or were unfit for curative-intent therapy: a phase 1 study. J. Urol. 209, 890–900 (2023).
Narayan, V. M. et al. Mechanism of action of nadofaragene firadenovec-vncg. Front. Oncol. 14, 1359725 (2024).
Boorjian, S. A. et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 22, 107–117 (2021).
US National Library of Medicine. ClinicalTrials.gov https://Clinicaltrials.Gov/Study/NCT06510374 (2025).
Svatek, R., Bivalacqua, T. & Daneshmand, S. PIVOT-006: A phase 3, randomized study of cretostimogene grenadenorepvec versus observation for the treatment of intermediate risk non-muscle invasive bladder cancer (IR-NMIBC) following transurethral resection of bladder tumor (TURBT). J. Clin. Oncol. 42, 4.
US National Library of Medicine. ClinicalTrials.gov https://Clinicaltrials.Gov/Study/NCT06111235 (2025).
Billerey, C. et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am. J. Pathol. 158, 1955–1959 (2001).
Valenza, C. et al. Emerging treatment landscape of non-muscle invasive bladder cancer. Expert. Opin. Biol. Ther. 22, 717–734 (2022).
Loriot, Y. et al. Erdafitinib or chemotherapy in advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 389, 1961–1971 (2023).
US National Library of Medicine. ClinicalTrials.gov https://Clinicaltrials.Gov/Study/NCT02202044 (2019).
US National Library of Medicine. ClinicalTrials.gov https://Clinicaltrials.Gov/Study/NCT06768346 (2025).
US National Library of Medicine. ClinicalTrials.gov https://Clinicaltrials.Gov/Study/NCT00595088 (2019).
US National Library of Medicine. ClinicalTrials.gov https://Clinicaltrials.Gov/Study/NCT05243550 (2024).
US National Library of Medicine. ClinicalTrials.gov https://Clinicaltrials.Gov/Study/NCT02720367 (2020).
US National Library of Medicine. ClinicalTrials.gov https://Clinicaltrials.Gov/Study/NCT05316155 (2025).
US National Library of Medicine. ClinicalTrials.gov https://Clinicaltrials.Gov/Study/NCT03914794 (2025).
US National Library of Medicine. ClinicalTrials.gov https://Clinicaltrials.Gov/Study/NCT03167151 (2020).
Author information
Authors and Affiliations
Contributions
L.W., H.M. and R.L. researched data for the article. All authors contributed substantially to discussion of the content. L.W., H.M. and R.L. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
S.P.L declares patent: TCGA classifier; clinical trials: Aura Bioscience, FKD, JBL (SWOG), Merck (Alliance), QED Therapeutics, Surge Therapeutics; advisory board/consulting fee: Aura Bioscience, Astra Zeneca, BMS, Pfizer/EMD Serono, Protara, Surge Therapeutics, Immunity Bio UroGen, Verity, Gilead, FKD, Viventa; honoraria: Grand Rounds Urology, UroToday. J.J.M. declares advisory boards/consulting: Merck, AstraZeneca, Janssen, BMS, UroGen, Prokarium, Imvax, Pfizer, Seagen/Astellas, Ferring, CG Oncology, Calibr, Immunity Bio, Protara, Photocure. S.P.P. declares research funding: National Institute on Aging, Bladder Cancer Advocacy Network, PRIME Education, Inc, Janssen; guidelines committee: American Urological Association: Upper Tract Urothelial Carcinoma Guidelines 2023 and AUA Practice Guidelines Committee; advisory/consulting: Janssen (SunRise-4 Global Co-PI), Immunity Bio, Merck, CG Oncology, Pfizer; editorial boards: European Urology, Bladder Cancer; steering committees/leadership: Bladder Cancer Advocacy Network, KCCure/Kidney Cancer Association/International Kidney Cancer Society; educational company presentations given: PeerView, MedScape, UroToday. A.M.K. declares patent: CyPRIT (Anderson Cancer Center #00043705); grants/contracts: FKD Therapies, Patient-Centered Outcomes Institute (PCORI), Photocure, Seagen, EnGene, Arquer Diagnostis, SWOG; advisory board/consulting: Astellas Pharma, Atonco Pharma, Biologic Dynamics, Bristol-Myers Squibb, CG Oncology, Cystotech, Eisai, EnGene, Ferring, Genentech, Imagin Medical, ImmunityBio, Imvax, Incyte, Janssen, Medac, Merk, Nonagen Bioscience, Pfizer, Photocure, Protara Therapeutics, Roche, Seagen, Sesen Bio, Theralase, urogen Pharma, US Biotest, Valar Labs, Vivet Therapeutics; boards/committee: IBCG, European Urology Oncology, Journal of Urology, UroToday, World Bladder Cancer patient Coalition, American Urological Association. L.D. declares funding agreement: 2i Genomics, Veracyte, Natera, AstraZeneca, Photocure and Ferring; advisory/consulting: Ferring, MSD, Cystotech, AstraZeneca and UroGen; honoraria: AstraZeneca, Pfizer and Roche and travel support from MSD. R.L. declares research support: Predicine; Valar labs; Johnson & Johnson; scientific adviser/consultant: BMS, Merck, CG Oncology, ImmunityBio, Pfizer, Johnson & Johnson, AstraZeneca, enGene, Valar Labs; honoraria: UroToday, IBCG, MashUP Media, MJH Lifesciences; travel: Predicine, CG Oncology, Johnson & Johnson. P.E.S. declares vice-chair of the NCCN bladder and penile cancer panel. All other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks Marco Paciotti and Xuan-Mei Piao for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wen, L., Miyagi, H., Spiess, P.E. et al. Low-grade non-muscle-invasive bladder cancer: molecular landscape, treatment strategies and emerging therapies. Nat Rev Urol 22, 846–861 (2025). https://doi.org/10.1038/s41585-025-01072-0
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41585-025-01072-0