Abstract
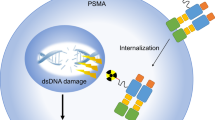
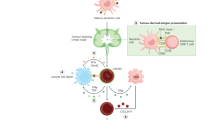
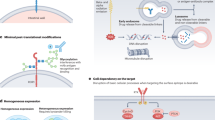
Prostate-specific membrane antigen (PSMA) is upregulated in prostate cancer cells relative to other cells. The increased expression and enzymatic activity of PSMA in high-stage disease confers a selective advantage on such cells, contributing to their increased proliferation, the tendency to metastasize and the development of a castration-resistant phenotype. The decades of radiobiochemical advances in the development of PSMA targeting radiolabelled ligands has led to the subsequent FDA approval of a number of radiotracers and radiotherapeutics for clinical use. Novel developments in therapeutic advances using PSMA-based combinatorial approaches include PSMA-targeted antibody–drug conjugates and PSMA-targeted radionuclide payloads. Combining and sequencing some of these strategies with standard therapy options such as surgery, radiotherapy and androgen receptor pathway inhibitors could improve patient outcomes. Immunotherapy and chimeric antigen receptor T cell therapy are relevant to PSMA-based therapies as PSMA can serve as a specific target antigen for these treatments, enabling precise tumour recognition and enhanced efficacy of prostate cancer therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
European Association of Urology. Epidemiology and aetiology. EAU https://uroweb.org/guidelines/prostate-cancer/chapter/epidemiology-and-aetiology (2023).
World Health Organization. Cancer today. IARC https://gco.iarc.fr/today/en/dataviz/tables?mode=population&types=1&cancers=27&group_populations=0&multiple_populations=1&sexes=1 (2022).
Rönningås, U., Holm, M., Fransson, P., Beckman, L. & Wennman-Larsen, A. Symptoms and quality of life among men starting treatment for metastatic castration-resistant prostate cancer — a prospective multicenter study. BMC Palliat. Care 23, 80 (2024).
Jain, R. et al. Patient, caregiver experiences in metastatic castration-resistant prostate cancer: insights from a multi-national survey. Future Oncol. 21, 2053–2066 (2025).
Rajasekaran, A. K., Anilkumar, G. & Christiansen, J. J. Is prostate-specific membrane antigen a multifunctional protein? Am. J. Physiol. Cell Physiol. 288, 975 (2005).
Bravaccini, S. et al. PSMA expression: a potential ally for the pathologist in prostate cancer diagnosis. Sci. Rep. 8, 4254 (2018).
Bakht, M. K. et al. Neuroendocrine differentiation of prostate cancer leads to PSMA suppression. Endocr. Relat. Cancer 26, 131–146 (2018).
Hupe, M. C. et al. Expression of Prostate-Specific Membrane Antigen (PSMA) on biopsies is an independent risk stratifier of prostate cancer patients at time of initial diagnosis. Front. Oncol. 8, 623 (2018).
[No authors listed] FDA approves first PSMA-targeted PET drug. J. Nucl. Med. 62, 11N (2021).
US Food and Drug Administration. FDA approves second PSMA-targeted PET imaging drug for men with prostate cancer. FDA https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-second-psma-targeted-pet-imaging-drug-men-prostate-cancer (2021).
ASCO Post Staff. FDA approves flotufolastat fluorine-18 injection, first radiohybrid PSMA-targeted PET imaging agent for prostate cancer. ASCO Post https://ascopost.com/news/may-2023/fda-approves-flotufolastat-fluorine-18-injection-first-radiohybrid-psma-targeted-pet-imaging-agent-for-prostate-cancer (2023).
Wurzer, A. et al. Radiohybrid ligands: a novel tracer concept exemplified by 18F- or 68Ga-labeled rhPSMA inhibitors. J. Nucl. Med. 61, 735–742 (2020).
Rauscher, I. et al. Detection efficacy of 18F-rhPSMA-7.3 PET/CT and impact on patient management in patients with biochemical recurrence of prostate cancer after radical prostatectomy and prior to potential salvage treatment. J. Nucl. Med. 62, 1719–1726 (2021).
Wang, Q. et al. Diagnostic efficacy of [99mTc]Tc-PSMA SPECT/CT for prostate cancer: a meta-analysis. BMC Cancer 24, 982 (2024).
de Barros, H. A. et al. Robot-assisted prostate-specific membrane antigen-radioguided salvage surgery in recurrent prostate cancer using a DROP-IN gamma probe: the first prospective feasibility study. Eur. Urol. 82, 97–105 (2022).
Medicines and Healthcare products Regulatory Agency. World first as MHRA approves trofolastat for diagnostic imaging of prostate cancer in men. MHRA https://www.gov.uk/government/news/world-first-as-mhra-approves-trofolastat-for-diagnostic-imaging-of-prostate-cancer-in-men (2025).
Pant, V., Vinjamuri, S., Zanial, A. Z. & Naeem, F. Lessons from a 3-year review of PSMA PET-CT in a tertiary setting: can we fine tune referral criteria by identifying factors predicting positivity and negativity? Diagnostics 13, 2542 (2023).
Udovicich, C. et al. Evolving paradigms in prostate cancer: the integral role of prostate-specific membrane antigen positron emission tomography/computed tomography in primary staging and therapeutic decision-making. Int. J. Radiat. Oncol. Biol. Phys. 121, 307–316 (2025).
Donswijk, M. L. et al. The accuracy and intra- and interobserver variability of PSMA PET/CT for the local staging of primary prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 51, 1741–1752 (2024).
National Center for Biotechnology Information. Molecular Imaging and Contrast Agent Database (MICAD) (NCBI, 2013).
Chang, S. S. Overview of prostate-specific membrane antigen. Rev. Urol. 6, S13–S18 (2004).
Sengupta, S., Asha Krishnan, M., Chattopadhyay, S. & Chelvam, V. Comparison of prostate-specific membrane antigen ligands in clinical translation research for diagnosis of prostate cancer. Cancer Rep. 2, e1169 (2019).
Stein, M. N. et al. Preliminary results from a phase 1/2 study of co-stimulatory bispecific PSMAxCD28 antibody REGN5678 in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 41, 154 (2023).
Kelly, W. et al. A phase 1/2 study of REGN4336, a PSMAxCD3 bispecific antibody, alone and in combination with cemiplimab in patients with metastatic castration-resistant prostate cancer. J. Clin. Oncol. 41, TPS284 (2023).
Hummel, H.-D. et al. Pasotuxizumab, a BiTE immune therapy for castration-resistant prostate cancer: phase I, dose-escalation study findings. Immunotherapy 13, 125–141 (2021).
Vaishampayan, U. N. et al. Phase II trial of pembrolizumab and anti-CD3 x anti-HER2 bispecific antibody-armed activated T cells in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 29, 122–133 (2023).
Milowsky, M. I. et al. Phase 1/2 multiple ascending dose trial of the prostate-specific membrane antigen-targeted antibody drug conjugate MLN2704 in metastatic castration-resistant prostate cancer. Urol. Oncol. 34, 530.e15–530.e21 (2016).
Paschalis, A. et al. Prostate-specific membrane antigen heterogeneity and DNA repair defects in prostate cancer. Eur. Urol. 76, 469–478 (2019).
Narayan, V. et al. PSMA-targeting TGFβ-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat. Med. 28, 724–734 (2022).
Alpen, E. L. in Radiation Biophysics 2nd edn 1–10 (Academic, 1998).
Kassis, A. I. Therapeutic radionuclides: biophysical and radiobiologic principles. Semin. Nucl. Med. 38, 358–366 (2008).
Kim, Y.-S. & Brechbiel, M. W. An overview of targeted alpha therapy. Tumor Biol. 33, 573–590 (2012).
Graf, F. et al. DNA double strand breaks as predictor of efficacy of the alpha-particle emitter Ac-225 and the electron emitter Lu-177 for somatostatin receptor targeted radiotherapy. PLoS ONE 9, e88239 (2014).
Batra, J. S. et al. Phase I trial of docetaxel plus lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 (177Lu-J591) for metastatic castration-resistant prostate cancer. Urol. Oncol. 38, 848.e9–848.e16 (2020).
Czerwińska, M., Bilewicz, A., Kruszewski, M., Wegierek-Ciuk, A. & Lankoff, A. Targeted radionuclide therapy of prostate cancer-from basic research to clinical perspectives. Molecules 25, 1743 (2020).
de Kruijff, R., Wolterbeek, H. & Denkova, A. A critical review of alpha radionuclide therapy — how to deal with recoiling daughters? Pharmaceuticals 8, 321–336 (2015).
Eychenne, R., Chérel, M., Haddad, F., Guérard, F. & Gestin, J.-F. Overview of the most promising radionuclides for targeted alpha therapy: the “Hopeful Eight”. Pharmaceutics 13, 906 (2021).
Golan, S. et al. Neoadjuvant 177Lu-PSMA-I&T radionuclide treatment in patients with high-risk prostate cancer before radical prostatectomy: a single-arm phase 1 trial. Eur. Urol. Oncol. 6, 151–159 (2023).
Griffiths, M. R. et al. First-in-human 212Pb-PSMA-targeted α-therapy SPECT/CT imaging in a patient with metastatic castration-resistant prostate cancer. J. Nucl. Med. 65, 664 (2024).
ARTBIO. FogPharma and ARTBIO announce collaboration to co-develop multiple Helicon-enabled alpha-particle radioligand therapies for the treatment of cancer. ARTBIO https://artbio.com/fogpharma-and-artbio-announce-collaboration-to-co-develop-multiple-helicon-enabled-alpha-particle-radioligand-therapies-for-the-treatment-of-cancer (2024).
Saidi, A., Stallons, T. A., Wong, A. G. & Torgue, J. J. Preclinical investigation of [212Pb]Pb-DOTAM-GRPR1 for peptide receptor radionuclide therapy in a prostate tumor model. J. Nucl. Med. 65, 1769–1775 (2024).
Oranomed. A diverse pipeline of 212Pb-labeled compounds. Oranomed https://www.oranomed.com/en/pipeline (2025).
Tagawa, S. T. et al. Prostate-specific membrane antigen-targeting alpha emitter via antibody delivery for metastatic castration-resistant prostate cancer: a phase I dose-escalation study of 225Ac-J591. J. Clin. Oncol. 42, 842–851 (2024).
Nauseef, J. T. et al. A phase I/II dose-escalation study of fractionated 225Ac-J591 for progressive metastatic castration-resistant prostate cancer (mCRPC) in patients with prior treatment with 177Lu-PSMA. J. Clin. Oncol. 41, TPS288 (2023).
Clarke, A. FDA clears IND for 225Ac-J591 in advanced prostate cancer. Urology Times https://www.urologytimes.com/view/fda-clears-ind-for-225ac-j591-in-advanced-prostate-cancer (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04946370 (2025).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04597411 (2025).
Sun, M. P. et al. Phase I results of a phase I/II study of pembrolizumab and AR signaling inhibitor (ARSI) with 225Ac-J591. J. Clin. Oncol. 41, 181–181 (2023).
Ling, S. W. et al. Evaluation of the tolerability and safety of [225Ac]Ac-PSMA-I&T in patients with metastatic prostate cancer: a phase I dose escalation study. BMC Cancer 24, 146 (2024).
Full-Life Technologies. Full-life technologies granted FDA fast track designation for 225Ac-FL-020 for the treatment of metastatic castration-resistant prostate cancer. Full-Life Technologies https://www.full-life.com/media/press-releases/27 (2024).
Hammer, S. et al. Preclinical efficacy of a PSMA-targeted thorium-227 conjugate (PSMA-TTC), a targeted alpha therapy for prostate cancer. Clin. Cancer Res. 26, 1985–1996 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03724747 (2024).
Delpassand, I. S. et al. Preliminary efficacy and safety results from the TATCIST trial: a PSMA-directed targeted alpha therapy with FPI-2265 (225Ac-PSMA-I&T) for the treatment of metastatic castration-resistant prostate cancer. Presented at the American Association for Cancer Research Annual Meeting (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05983198 (2025).
Saleh, R. et al. ACCEL: [Ac-225]-PSMA-62 phase Ia/Ib/II clinical trial to characterize efficacy, safety, tolerability, and dosimetry in oligometastatic hormone-sensitive and metastatic castration-resistant prostate cancer. J. Clin. Oncol. 43, 842–851 (2025).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06881823 (2025).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06855277 (2025).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06402331 (2025).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06780670 (2025).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04225910 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05902247 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06217822 (2025).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06052306 (2025).
Klaassen, Z. PSMA and beyond 2025: overview of clinical trials of PSMA RLT in 2025. UroToday https://www.urotoday.com/conference-highlights/2025-ucsf-ucla-psma-conference/159332-psma-and-beyond-2025-overview-of-clinical-trials-of-psma-rlt-in-2025.html (2025).
Sgouros, G., Bodei, L., McDevitt, M. R. & Nedrow, J. R. Radiopharmaceutical therapy in cancer: clinical advances and challenges. Nat. Rev. Drug Discov. 19, 589–608 (2020).
US Food and Drug Administration. FDA approves Pluvicto for metastatic castration-resistant prostate cancer. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pluvicto-metastatic-castration-resistant-prostate-cancer (2022).
Hennrich, U. & Eder, M. [177Lu]Lu-PSMA-617 (Pluvicto): the first FDA-approved radiotherapeutical for treatment of prostate cancer. Pharmaceuticals 15, 1292 (2022).
Tagawa, S. T. et al. Randomized, double-blinded phase II study of ketoconazole (keto), hydrocortisone (HC), and anti-PSMA antibody J591 labeled with 177Lu or 111In in patients (pts) with high-risk non-metastatic (met) castration-resistant prostate cancer (M0 CRPC). J. Clin. Oncol. 41, LBA21 (2023).
Tagawa, S. T. et al. Phase 1/2 study of fractionated dose lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 (177 Lu-J591) for metastatic castration-resistant prostate cancer. Cancer 125, 2561–2569 (2019).
Bander, N. H. et al. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J. Clin. Oncol. 23, 4591–4601 (2005).
Tagawa, S. T. et al. Anti-prostate specific membrane antigen-based radioimmunotherapy for prostate. Cancer 116, 1075–1083 (2010).
Liu, H. et al. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res. 57, 3629–3634 (1997).
Fung, E. K. et al. Targeting of radiolabeled J591 antibody to PSMA-expressing tumors: optimization of imaging and therapy based on non-linear compartmental modeling. EJNMMI Res. 6, 7 (2016).
Tagawa, S. T. et al. Phase II study of lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin. Cancer Res. 19, 5182–5191 (2013).
National Cancer Institute. Lutetium Lu 177 rsopatamab tetraxetan. NCI https://www.cancer.gov/publications/dictionaries/cancer-drug/def/lutetium-lu-177-rosopatamab-tetraxetan (2023).
Telix Pharmaceuticals. First patient dosed in phase III ProstACT GLOBAL study of antibody-based prostate cancer therapy candidate, TLX591. TelixPharmaceuticals https://telixpharma.com/news-views/first-patient-dosed-in-phase-iii-prostact-global-study-of-antibody-based-prostate-cancer-therapy-candidate-tlx591 (2023).
Telix Pharmaceuticals. Telix announces positive rPFS data from ProstACT SELECT trial of TLX591 rADC therapy candidate in prostate cancer. PR Newswire https://www.prnewswire.com/apac/news-releases/telix-announces-positive-rpfs-data-from-prostact-select-trial-of-tlx591-radc-therapy-candidate-in-prostate-cancer-302160140.html (2024).
Kassis, A. I. Cancer therapy with Auger electrons: are we almost there? J. Nucl. Med. 44, 1479–1481 (2003).
Bodei, L., Kassis, A. I., Adelstein, S. J. & Mariani, G. Radionuclide therapy with iodine-125 and other auger-electron-emitting radionuclides: experimental models and clinical applications. Cancer Biother. Radiopharm. 18, 861–877 (2003).
Müller, C. et al. Terbium-161 for PSMA-targeted radionuclide therapy of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 46, 1919–1930 (2019).
Buteau, J. P. et al. First-in-human results of terbium-161 [161Tb]Tb-PSMA-I&T dual beta–Auger radioligand therapy in patients with metastatic castration-resistant prostate cancer (VIOLET): a single-centre, single-arm, phase 1/2 study. Lancet Oncol. 26, 1009–1017 (2025).
Chirindel, A. et al. First-in-human administration of [161Tb]Tb-SibuDAB and comparative dosimetry with standard [177Lu]Lu-PSMA-I&T as part of the PROGNOSTICS phase Ia study. Eur. J. Nucl. Med. Mol. Imaging 52, 1628–1630 (2025).
Fu, Z., Li, S., Han, S., Shi, C. & Zhang, Y. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal. Transduct. Target. Ther. 7, 1–25 (2022).
de Bono, J. S. et al. Phase I study of MEDI3726: a prostate-specific membrane antigen-targeted antibody–drug conjugate, in patients with mCRPC after failure of abiraterone or enzalutamide. Clin. Cancer Res. 27, 3602–3609 (2021).
Shen, J. et al. 1804P APEX-01: first-in-human phase I/II study of ARX517 an anti- prostate-specific membrane antigen (PSMA) antibody-drug conjugate (ADC) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). Ann. Oncol. 34, S974–S975 (2023).
Chu, Y., Zhou, X. & Wang, X. Antibody-drug conjugates for the treatment of lymphoma: clinical advances and latest progress. J. Hematol. Oncol. 14, 88 (2021).
Li, F. et al. Intracellular released payload influences potency and bystander-killing effects of antibody-drug conjugates in preclinical models. Cancer Res. 76, 2710–2719 (2016).
Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2-Positive breast cancer. N. Engl. J. Med. 382, 610–621 (2020).
Hurvitz, S. A. et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet 401, 105–117 (2023).
US Food and Drug Administraton. FDA approves fam-trastuzumab deruxtecan-nxki for unresectable or metastatic HER2-positive breast cancer. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fam-trastuzumab-deruxtecan-nxki-unresectable-or-metastatic-her2-positive-breast-cancer (2019).
Daiichi-Sankyo. ENHERTU approved in the US as first tumor agnostic HER2 directed therapy for previously treated patients with metastatic HER2 positive solid tumors. Daiichi-Sankyo https://daiichisankyo.us/press-releases/-/article/enhertu-approved-in-the-u-s-as-first-tumor-agnostic-her2-directed-therapy-for-previously-treated-patients-with-metastatic-her2-positive-solid-tumors (2024).
ecancer. FDA approves new treatment option for patients with HER2-positive breast cancer who have progressed on available therapies. ecancer https://ecancer.org/en/news/17123-fda-approves-new-treatment-option-for-patients-with-her2-positive-breast-cancer-who-have-progressed-on-available-therapies (2020).
Cho, S. et al. Antitumor activity of MEDI3726 (ADCT-401), a pyrrolobenzodiazepine antibody–drug conjugate targeting PSMA, in preclinical models of prostate cancer. Mol. Cancer Ther. 17, 2176–2186 (2018).
Petrylak, D. P. et al. Phase 1 study of PSMA ADC, an antibody-drug conjugate targeting prostate-specific membrane antigen, in chemotherapy-refractory prostate cancer. Prostate 79, 604–613 (2019).
Petrylak, D. P. et al. PSMA ADC monotherapy in patients with progressive metastatic castration-resistant prostate cancer following abiraterone and/or enzalutamide: efficacy and safety in open-label single-arm phase 2 study. Prostate 80, 99–108 (2020).
Colombo, R. & Rich, J. R. The therapeutic window of antibody drug conjugates: a dogma in need of revision. Cancer Cell 40, 1255–1263 (2022).
Skidmore, L. et al. Preclinical characterization of ARX517, a next-generation anti-PSMA antibody drug conjugate for the treatment of metastatic castration-resistant prostate cancer [abstract 3997]. Cancer Res. 83, 3997 (2023).
Ambrx Biopharma. FDA grants fast track designation for Ambrx’s ARX517 for the treatment of metastatic castration-resistant prostate cancer. GlobeNewswire https://www.globenewswire.com/news-release/2023/07/19/2707358/0/en/FDA-Grants-Fast-Track-Designation-for-Ambrx-s-ARX517-for-the-Treatment-of-Metastatic-Castration-Resistant-Prostate-Cancer.html (2023).
Klaassen, Z. APEX-01: first-in-human phase 1/2 study of ARX517, an anti-PSMA ADC, in patients with mCRPC. UroToday https://www.urotoday.com/conference-highlights/esmo-2023/esmo-2023-prostate-cancer/147612-esmo-2023-apex-01-first-in-human-phase-1-2-study-of-arx517-an-anti-psma-adc-in-patients-with-mcrpc.html (2023).
Skidmore, L. K. et al. Preclinical characterization of ARX517, a site-specific stable PSMA-targeted antibody-drug conjugate for the treatment of metastatic castration-resistant prostate cancer. Mol. Cancer Ther. 23, 1842–1853 (2024).
Huehls, A. M., Coupet, T. A. & Sentman, C. L. Bispecific T-cell engagers for cancer immunotherapy. Immunol. Cell Biol. 93, 290–296 (2015).
Haber, L. et al. Generation of T-cell-redirecting bispecific antibodies with differentiated profiles of cytokine release and biodistribution by CD3 affinity tuning. Sci. Rep. 11, 14397 (2021).
Tian, Z., Liu, M., Zhang, Y. & Wang, X. Bispecific T cell engagers: an emerging therapy for management of hematologic malignancies. J. Hematol. Oncol. 14, 75 (2021).
US Food and Drug Administration. FDA grants accelerated approval to elranatamab-bcmm for multiple myeloma. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-elranatamab-bcmm-multiple-myeloma (2023).
US Food and Drug Administration. FDA grants regular approval to blinatumomab and expands indication to include Philadelphia chromosome-positive B cell. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-blinatumomab-and-expands-indication-include-philadelphia-chromosome (2017).
US Food and Drug Administration. FDA granted accelerated approval to blinatumomab (Blincyto, Amgen Inc.) for the treatment of adult and pediatric patients with B-cell precursor acute lymphoblastic leukemia. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-granted-accelerated-approval-blinatumomab-blincyto-amgen-inc-treatment-adult-and-pediatric (2018).
US Food and Drug Administration. FDA grants accelerated approval to tarlatamab-dlle for extensive stage small cell lung cancer. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-tarlatamab-dlle-extensive-stage-small-cell-lung-cancer (2024).
Ahn, M.-J. et al. Tarlatamab for patients with previously treated small-cell lung cancer. N. Engl. J. Med. 389, 2063–2075 (2023).
Deegen, P. et al. The PSMA-targeting half-life extended BiTE therapy AMG 160 has potent antitumor activity in preclinical models of metastatic castration-resistant prostate cancer. Clin. Cancer Res. 27, 2928–2937 (2021).
Tran, B. et al. Results from a phase I study of AMG 160, a half-life-extended (HLE), PSMA-targeted, bispecific T-cell engager (BiTE) immune therapy for metastatic castration-resistant prostate cancer (mCRPC) [abstract 609O]. Ann. Oncol. 31, S507 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04631601 (2025).
Dorff, T. et al. A phase I study of acapatamab, a half-life extended, PSMA-targeting bispecific T-cell engager for metastatic castration-resistant prostate cancer. Clin. Cancer Res. 30, 1488–1500 (2024).
Dang, K. et al. Attenuating CD3 affinity in a PSMAxCD3 bispecific antibody enables killing of prostate tumor cells with reduced cytokine release. J. Immunother. Cancer 9, e002488 (2021).
Falchook, G. S. et al. Phase 1 clinical trial of AMG 340, a prostate-specific membrane antigen (PSMA)-targeted T-cell engager with a novel low-affinity CD3 binding domain designed to mitigate toxicity for the treatment of metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 42, e14587 (2024).
Machulkin, A. E. et al. Synthesis, characterization, and preclinical evaluation of a small-molecule prostate-specific membrane antigen-targeted monomethyl auristatin E conjugate. J. Med. Chem. 64, 17123–17145 (2021).
Stein, M. N. et al. Pasritamig, a first-in-class, bispecific T-Cell engager targeting human kallikrein 2, in metastatic castration-resistant prostate cancer: a phase 1 study. J. Clin. Oncol. 43, 2515–2526 (2025).
Markowski, M. et al. Phase I study of CCW702, a bispecific small molecule-antibody conjugate targeting PSMA and CD3 in patients with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 39, TPS5094 (2021).
Austin, R. J. et al. TriTACs, a novel class of T-cell–engaging protein constructs designed for the treatment of solid tumors. Mol. Cancer Ther. 20, 109–120 (2021).
Molloy, M. E. et al. HPN328, a trispecific T cell–activating protein construct targeting DLL3-expressing solid tumors. Mol. Cancer Ther. 23, 1294–1304 (2024).
Bendell, J. C. et al. First-in-human phase I study of HPN424, a tri-specific half-life extended PSMA-targeting T-cell engager in patients with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 38, 5552–5552 (2020).
De, B. J. S. et al. Results of an ongoing phase 1/2a dose escalation study of HPN424, a tri-specific half-life extended PSMA-targeting T-cell engager, in patients with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 39, 5013 (2021).
Taylor, N. P. Regeneron rethinks CD28 bispecific R&D plan after 2 patients die. Fierce Biotech https://www.fiercebiotech.com/biotech/regeneron-rethinks-cd28-bispecific-rd-plan-after-2-patients-die (2023).
Amgen. Teneobio acquisition adds talent and new types of antibodies to Amgen. Amgen https://www.amgen.com/stories/2022/01/teneobio-acquisition-adds-talent-and-new-types-of-antibodies-to-amgen (2022).
US Food and Drug Administration. FDA approves tisagenlecleucel for relapsed or refractory follicular lymphoma. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tisagenlecleucel-relapsed-or-refractory-follicular-lymphoma (2022).
US Food and Drug Administration. FDA approves tisagenlecleucel for B-cell ALL and tocilizumab for cytokine release syndrome. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tisagenlecleucel-b-cell-all-and-tocilizumab-cytokine-release-syndrome (2017).
US Food and Drug Administration. YESCARTA. FDA https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/yescarta (2025).
US Food and Drug Administration. TECARTUS. FDA https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/tecartus (2025).
US Food and Drug Administration. FDA approves lisocabtagene maraleucel for relapsed or refractory mantle cell lymphoma. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lisocabtagene-maraleucel-relapsed-or-refractory-mantle-cell-lymphoma (2024).
US Food and Drug Administration. FDA approves idecabtagene vicleucel for multiple myeloma. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-idecabtagene-vicleucel-multiple-myeloma (2021).
US Food and Drug Administration. FDA approves ciltacabtagene autoleucel for relapsed or refractory multiple myeloma. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ciltacabtagene-autoleucel-relapsed-or-refractory-multiple-myeloma (2022).
Gade, T. P. F. et al. Targeted elimination of prostate cancer by genetically directed human T lymphocytes. Cancer Res. 65, 9080–9088 (2005).
Maher, J., Brentjens, R. J., Gunset, G., Rivière, I. & Sadelain, M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ/CD28 receptor. Nat. Biotechnol. 20, 70–75 (2002).
Junghans, R. P. et al. Phase I trial of anti-PSMA designer CAR-T cells in prostate cancer: possible role for interacting interleukin 2-T cell pharmacodynamics as a determinant of clinical response. Prostate 76, 1257–1270 (2016).
Slovin, S. F. et al. Phase 1 study of P-PSMA-101 CAR-T cells in patients with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 40, 98 (2022).
Rosa, K. FDA halts phase 1 trial with P-PSMA-101 in mCRPC following patient death. OncLive https://www.onclive.com/view/fda-halts-phase-1-trial-with-p-psma-01-in-mcrpc-following-patient-death (2020).
Yakoub-Agha, I. et al. Management of adults and children undergoing CAR T-cell therapy: best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE). Haematologica 105, 297–316 (2020).
eviQ. Cytokine-release syndrome (CRS). eviQ https://www.eviq.org.au/clinical-resources/oncological-emergencies/3500-cytokine-release-syndrome-associated-with-car#background (2025).
Saxena, A. et al. Prevalence of adverse events following T-cell engaging bispecific antibodies (bsAbs) and chimeric antigen receptor (CAR) T-cell therapy in patients with metastatic castrate-resistant prostate cancer (mCRPC): a meta-analysis. J. Clin. Oncol. 43, 128–128 (2025).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/study/NCT0308 (2025).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04227275 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04768608 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05732948 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04633148 (2024).
Kfoury, Y. et al. Human prostate cancer bone metastases have an actionable immunosuppressive microenvironment. Cancer Cell 39, 1464–1478.e8 (2021).
Maude, S. L. et al. Tisagenlecleucel in children and young adults with B-Cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 (2018).
Roddie, C. et al. Obecabtagene autoleucel in adults with B-cell acute lymphoblastic leukemia. N. Engl. J. Med. 391, 2219–2230 (2024).
Sartor, O. et al. Lutetium-177–PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 385, 1091–1103 (2021).
Abdel-Aty, H. et al. The STAMPEDE2 trial: a site survey of current patterns of care, access to imaging and treatment of metastatic prostate cancer. Clin. Oncol. 35, e628–e635 (2023).
STAMPEDE2. STAMPEDE2: a randomised controlled platform trial testing treatments in metastatic hormone sensitive prostate cancer. STAMPEDE2 https://www.stampede2trial.org (2025).
Davis, I. D. et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N. Engl. J. Med. 381, 121–131 (2019).
Armstrong, A. J. et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J. Clin. Oncol. 37, 2974–2986 (2019).
Smith, M. R. et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N. Engl. J. Med. 378, 1408–1418 (2018).
Hussain, M. et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N. Engl. J. Med. 378, 2465–2474 (2018).
Sternberg, C. N. et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N. Engl. J. Med. 382, 2197–2206 (2020).
Rice, M. A., Malhotra, S. V. & Stoyanova, T. Second-generation antiandrogens: from discovery to standard of care in castration resistant prostate cancer. Front. Oncol. 9, 801 (2019).
Chen, Y., Zhou, Q., Hankey, W., Fang, X. & Yuan, F. Second generation androgen receptor antagonists and challenges in prostate cancer treatment. Cell Death Dis. 13, 632 (2022).
Emmett, L. et al. [177Lu]Lu-PSMA-617 plus enzalutamide in patients with metastatic castration-resistant prostate cancer (ENZA-p): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 25, 563–571 (2024).
Yazdanpanah, O. et al. Efficacy of 177Lu-PSMA-617 with or without ARPIs for the treatment of mCRPC: VISION secondary analysis. J. Clin. Oncol. 43, 121–121 (2025).
Muniz, M. et al. Outcomes for 177Lu-PSMA-617 with and without concurrent use of ARPIs in patients with mCRPC. J. Clin. Oncol. 43, 112–112 (2025).
Morales, J. C. et al. Review of poly (ADP-ribose) polymerase (PARP) mechanisms of action and rationale for targeting in cancer and other diseases. Crit. Rev. Eukaryot. Gene Expr. 24, 15–28 (2014).
Mateo, J. et al. DNA-repair defects and olaparib in metastatic prostate cancer. N. Engl. J. Med. 373, 1697–1708 (2015).
Sandhu, S., Guo, C. & Hofman, M. S. Radionuclide therapy in prostate cancer: from standalone to combination PSMA theranostics. J. Nucl. Med. 62, 1660–1668 (2021).
Fenor de la Maza, M. D., Pérez Gracia, J. L., Miñana, B. & Castro, E. PARP inhibitors alone or in combination for prostate cancer. Ther. Adv. Urol. 16, 17562872241272928 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03874884 (2025).
Sandhu, S. et al. LuPARP: phase 1 trial of 177Lu-PSMA-617 and olaparib in patients with metastatic castration resistant prostate cancer (mCRPC). J. Clin. Oncol. 41, 5005 (2023).
Ruigrok, E. A. M. et al. Preclinical assessment of the combination of PSMA-targeting radionuclide therapy with PARP inhibitors for prostate cancer treatment. Int. J. Mol. Sci. 23, 8037 (2022).
Connell-Crowley, L., Harper, J. W. & Goodrich, D. W. Cyclin D1/Cdk4 regulates retinoblastoma protein-mediated cell cycle arrest by site-specific phosphorylation. Mol. Biol. Cell 8, 287–301 (1997).
Franco, R., Cao, J. Q., Yassa, M. & Hijal, T. Safety of CDK4/6 inhibitors combined with radiotherapy in patients with metastatic breast cancer: a review of the literature. Curr. Oncol. 30, 5485–5496 (2023).
Kubeczko, M. et al. Safety and feasibility of radiation therapy combined with CDK 4/6 inhibitors in the management of advanced breast cancer. Cancers 15, 690 (2023).
O’Leary, B., Finn, R. S. & Turner, N. C. Treating cancer with selective CDK4/6 inhibitors. Nat. Rev. Clin. Oncol. 13, 417–430 (2016).
Von Hoff, D. D. et al. Phase I study of PSMA-targeted docetaxel-containing nanoparticle BIND-014 in patients with advanced solid tumors. Clin. Cancer Res. 22, 3157–3163 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05113537 (2025).
Pienta, K. J. Preclinical mechanisms of action of docetaxel and docetaxel combinations in prostate cancer. Semin. Oncol. 28, 3–7 (2001).
Puente, J. et al. Docetaxel in prostate cancer: a familiar face as the new standard in a hormone-sensitive setting. Ther. Adv. Med. Oncol. 9, 307–318 (2017).
Dhiantravan, N. et al. UpFrontPSMA: a randomized phase 2 study of sequential 177Lu-PSMA-617 and docetaxel vs docetaxel in metastatic hormone-naïve prostate cancer (clinical trial protocol). BJU Int. 128, 331–342 (2021).
Azad, A. A. et al. Sequential [177Lu]Lu-PSMA-617 and docetaxel versus docetaxel in patients with metastatic hormone-sensitive prostate cancer (UpFrontPSMA): a multicentre, open-label, randomised, phase 2 study. Lancet Oncol. 25, 1267–1276 (2024).
Wei, X., Schlenkhoff, C., Schwarz, B., Essler, M. & Ahmadzadehfar, H. Combination of 177Lu-PSMA-617 and external radiotherapy for the treatment of cerebral metastases in patients with castration-resistant metastatic prostate cancer. Clin. Nucl. Med. 42, 704–706 (2017).
Waldman, A. D., Fritz, J. M. & Lenardo, M. J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 20, 651–668 (2020).
Drake, C. G., Lipson, E. J. & Brahmer, J. R. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nat. Rev. Clin. Oncol. 11, 24–37 (2014).
Haffner, M. C. et al. Genomic and phenotypic heterogeneity in prostate cancer. Nat. Rev. Urol. 18, 79–92 (2021).
Vareki, S. M. High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. J. Immunother. Cancer 6, 157 (2018).
Verma, S., Turkbey, B. & Muradyan, N. Overview of dynamic contrast-enhanced MRI in prostate cancer diagnosis and management. AJR Am. J. Roentgenol. 198, 1277 (2012).
Dunn, G. P., Bruce, A. T., Ikeda, H., Old, L. J. & Schreiber, R. D. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 3, 991–998 (2002).
Sharma, P. & Allison, J. P. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161, 205–214 (2015).
Antonarakis, E. S. et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J. Clin. Oncol. 38, 395–405 (2019).
Powles, T. et al. Atezolizumab with enzalutamide versus enzalutamide alone in metastatic castration-resistant prostate cancer: a randomized phase 3 trial. Nat. Med. 28, 144–153 (2022).
Petrylak, D. P. et al. Pembrolizumab plus docetaxel versus docetaxel for previously treated metastatic castration-resistant prostate cancer: the randomized, double-blind, phase III KEYNOTE-921 trial. J. Clin. Oncol. 43, 1638–1649 (2025).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05806814 (2025).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05568550 (2025).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05077098 (2025).
Cheema, A. K. et al. Radiotherapy induces innate immune responses in patients treated for prostate cancers. Clin. Cancer Res. 29, 921–929 (2023).
Eapen, R. S. et al. Neoadjuvant lutetium PSMA, the TIME and immune response in high-risk localized prostate cancer. Nat. Rev. Urol. 21, 676–686 (2024).
Lunj, S. et al. Immune effects of α and β radionuclides in metastatic prostate cancer. Nat. Rev. Urol. 21, 651–661 (2024).
Colciago, R. R. et al. Overview of the synergistic use of radiotherapy and immunotherapy in cancer treatment: current challenges and scopes of improvement. Expert Rev. Anticancer Ther. 23, 135–145 (2023).
Mole, R. H. Whole body irradiation; radiobiology or medicine? Br. J. Radiol. 26, 234–241 (1953).
Demaria, S. et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 58, 862–870 (2004).
Sandhu, S. et al. Evolution: phase II study of radionuclide 177Lu-PSMA-617 therapy versus 177Lu-PSMA-617 in combination with ipilimumab and nivolumab for men with metastatic castration-resistant prostate cancer (mCRPC; ANZUP 2001). J. Clin. Oncol. 41, TPS271 (2023).
Sandhu, S. et al. PRINCE: phase I trial of 177 Lu-PSMA-617 in combination with pembrolizumab in patients with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 40, 5017 (2022).
Aggarwal, R. et al. Single-dose 177Lu-PSMA-617 followed by maintenance pembrolizumab in patients with metastatic castration-resistant prostate cancer: an open-label, dose-expansion, phase 1 trial. Lancet Oncol. 24, 1266–1276 (2023).
Sayyid, R. The current state of treatment implementation for mCRPC in North America. UroToday https://www.urotoday.com/library-resources/advanced-prostate-cancer/147780-the-current-state-of-treatment-implementation-for-mcrpc-in-north-america.html (2023).
Pilepich, M. V. et al. Phase III radiation therapy oncology group (RTOG) trial 86-10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int. J. Radiat. Oncol. Biol. Phys. 50, 1243–1252 (2001).
Roach, M. et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J. Clin. Oncol. 26, 585–591 (2008).
Denham, J. W. et al. Short-term androgen deprivation and radiotherapy for locally advanced prostate cancer: results from the Trans-Tasman Radiation Oncology Group 96.01 randomised controlled trial. Lancet Oncol. 6, 841–850 (2005).
Dhiantravan, N. et al. Clinical trial protocol for LuTectomy: a single-arm study of the dosimetry, safety, and potential benefit of 177Lu-PSMA-617 prior to prostatectomy. Eur. Urol. Focus 7, 234–237 (2021).
Jones, C. U. et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N. Engl. J. Med. 365, 107–118 (2011).
National Institute for Health and Care Excellence. Locally Advanced Prostate Cancer (NICE, 2019).
European Association of Urology. Prostace cancer — treatment. EAU https://uroweb.org/guidelines/prostate-cancer/chapter/treatment (2025).
Klaassen, Z. EAU 2023: LuTectomy: a prospective study of dosimetry, safety and potential benefit of upfront 177Lu-PSMA-617 radioligand therapy prior to radical prostatectomy in men with high-risk localized prostate cancer. UroToday https://www.urotoday.com/conference-highlights/eau-annual-congress-2023/eau-2023-prostate-cancer/143074-eau-2023-lutectomy-a-prospective-study-of-dosimetry-safety-and-potential-benefit-of-upfront-177lu-psma-617-radioligand-therapy-prior-to-radical-prostatectomy-in-men-with-high-risk-localized-prostate-cancer.html (2023).
Eapen, R. S. et al. Administering [177Lu]Lu-PSMA-617 prior to radical prostatectomy in men with high-risk localised prostate cancer (LuTectomy): a single-centre, single-arm, phase 1/2 study. Eur. Urol. 85, 217–226 (2024).
Adeleke, S., Haroon, A. & Kasivisvanathan, V. Re: [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Eur. Urol. 80, 118–119 (2021).
Bao, X. & Xie, L. Targeting purinergic pathway to enhance radiotherapy-induced immunogenic cancer cell death. J. Exp. Clin. Cancer Res. 41, 222 (2022).
Chen, C., Liu, Y. & Cui, B. Effect of radiotherapy on T cell and PD-1 / PD-L1 blocking therapy in tumor microenvironment. Hum. Vaccin. Immunother. 17, 1555–1567 (2021).
Krafft, U. et al. A randomized phase I/II study of neoadjuvant treatment with 177-Lutetium-PSMA-617 with or without ipilimumab in patients with very high-risk prostate cancer who are candidates for radical prostatectomy (NEPI trial). J. Clin. Oncol. 42, TPS352 (2024).
Chen, J. et al. Synergistic effects of immunotherapy and adjunctive therapies in prostate cancer management. Crit. Rev. Oncol. Hematol. 207, 104604 (2025).
Pisansky, T. M., Thompson, I. M., Valicenti, R. K., D’Amico, A. V. & Selvarajah, S. Adjuvant and salvage radiotherapy after prostatectomy: ASTRO/AUA guideline amendment 2018–2019. J. Urol. 202, 533–538 (2019).
George, G. et al. Long-term adherence to GnRH agonists in men with prostate cancer: a nation-wide population-based study in prostate cancer data base Sweden. Scand. J. Urol. 54, 20–26 (2020).
von Eyben, F. E. et al. 177Lu-PSMA-617 radioligand therapy for a patient with lymph node metastatic prostate cancer. Oncotarget 8, 66112–66116 (2017).
Arham, A. B. et al. Updated follow up of a randomized, double-blinded phase II study of ketoconazole (keto), hydrocortisone (HC), and anti-PSMA antibody J591 labeled with 177Lu or 111In in patients (pts) with high-risk non-metastatic (met) castration-resistant prostate cancer (M0 CRPC). J. Clin. Oncol. 43, 172–172 (2025).
Sartor, A. et al. PSMAddition: a phase 3 trial to compare treatment with 177Lu-PSMA-617 plus standard of care (SOC) versus SOC alone in patients with metastatic hormone-sensitive prostate cancer. J. Clin. Oncol. 40, TPS210 (2022).
Petrelli, F. et al. Better survival of patients with oligo- compared with polymetastatic cancers: a systematic review and meta-analysis of 173 studies. F1000Res. 10, 423 (2021).
Palma, D. A. et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J. Clin. Oncol. 38, 2830–2838 (2020).
Wang, Y. et al. The future of PSMA PET and WB MRI as next-generation imaging tools in prostate cancer. Nat. Rev. Urol. 19, 475–493 (2022).
Ost, P. et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J. Clin. Oncol. 36, 446–453 (2018).
Nguyen, P. L. et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur. Urol. 67, 825–836 (2015).
Cao, B., Kim, M., Reizine, N. M. & Moreira, D. M. Adverse events and androgen receptor signaling inhibitors in the treatment of prostate cancer: a systematic review and multivariate network meta-analysis. Eur. Urol. Oncol. 6, 237–250 (2023).
Ma, T. M. et al. LUNAR: a randomized phase 2 study of 177Lutetium-PSMA neoadjuvant to ablative radiotherapy for oligorecurrent prostate cancer (clinical trial protocol). BJU Int. 132, 65–74 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05079698 (2025).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05560659 (2025).
Privé, B. M. et al. Lutetium-177-PSMA-617 in low-volume hormone-sensitive metastatic prostate cancer: a prospective pilot study. Clin. Cancer Res. 27, 3595–3601 (2021).
Telix Pharmaceuticals. ProstACT update: TARGET study ethics approval. Telix Pharmaceuticals https://telixpharma.com/news-views/prostact-update-target-study-ethics-approval (2022).
Privé, B. M. et al. Lutetium-177-PSMA-I&T as metastases directed therapy in oligometastatic hormone sensitive prostate cancer, a randomized controlled trial. BMC Cancer 20, 884 (2020).
Satapathy, S. et al. Short-course 177Lu-PSMA-617 radioligand therapy in high-volume metastatic hormone-sensitive prostate cancer: time to take the leap? Eur. Urol. 80, 390–392 (2021).
Pfannkuchen, N., Bausbacher, N., Pektor, S., Miederer, M. & Rosch, F. In vivo evaluation of [225Ac]Ac-DOTAZOL for α-Therapy of bone metastases. Curr. Radiopharm. 11, 223–230 (2018).
Chi, K. N. et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N. Engl. J. Med. 381, 13–24 (2019).
Fizazi, K. et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 20, 686–700 (2019).
Hofman, M. S. et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet 397, 797–804 (2021).
Kostos, L. et al. AlphaBet: combination of Radium-223 and [177Lu]Lu-PSMA-I&T in men with metastatic castration-resistant prostate cancer (clinical trial protocol). Front. Med. 9, 1059122 (2022).
Morris, M. J. et al. 177Lu-PSMA-617 versus a change of androgen receptor pathway inhibitor therapy for taxane-naive patients with progressive metastatic castration-resistant prostate cancer (PSMAfore): a phase 3, randomised, controlled trial. Lancet 404, 1227–1239 (2024).
US Food and Drug Administration. FDA expands Pluvicto’s metastatic castration-resistant prostate cancer indication. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-expands-pluvictos-metastatic-castration-resistant-prostate-cancer-indication (2025).
Gafita, A. et al. Predictors and real-world use of prostate-specific radioligand therapy: PSMA and beyond. Am. Soc. Clin. Oncol. Educ. Book 42, 1–17 (2022).
Roberts, M. J. et al. Using PSMA imaging for prognostication in localized and advanced prostate cancer. Nat. Rev. Urol. 20, 23–47 (2023).
Gafita, A. et al. Nomograms to predict outcomes after 177Lu-PSMA therapy in men with metastatic castration-resistant prostate cancer: an international, multicentre, retrospective study. Lancet Oncol. 22, 1115–1125 (2021).
Herrmann, K. et al. Multivariable models of outcomes with [177Lu]Lu-PSMA-617: analysis of the phase 3 VISION trial. EClinicalMedicine 77, 102862 (2024).
Gafita, A. et al. Early experience of rechallenge 177Lu-PSMA radioligand therapy after an initial good response in patients with advanced prostate cancer. J. Nucl. Med. 60, 644–648 (2019).
Seifert, R. et al. Safety and efficacy of extended therapy with [177Lu]Lu-PSMA: a German multicenter study. J. Nucl. Med. 65, 909–916 (2024).
Lawal, I. O. et al. Hematologic toxicity profile and efficacy of [225Ac]Ac-PSMA-617 α-radioligand therapy of patients with extensive skeletal metastases of castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 49, 3581–3592 (2022).
Khawar, A. et al. Preliminary results of biodistribution and dosimetric analysis of [68Ga]Ga-DOTAZOL: a new zoledronate-based bisphosphonate for PET/CT diagnosis of bone diseases. Ann. Nucl. Med. 33, 404–413 (2019).
Mazzucato, G. et al. Robot-assisted PSMA-radioguided salvage surgery for oligorecurrent prostate cancer using the novel SENSEI drop-in gamma probe: correlation of intraoperative measurements to preoperative imaging and final histology. Cancers 17, 93 (2024).
Gandaglia, G. et al. Prostate-specific membrane antigen radioguided surgery to detect nodal metastases in primary prostate cancer patients undergoing robot-assisted radical prostatectomy and extended pelvic lymph node dissection: results of a planned interim analysis of a prospective phase 2 study. Eur. Urol. 82, 411–418 (2022).
Oderda, M. et al. Robot-assisted PSMA-radioguided surgery to assess surgical margins and nodal metastases in prostate cancer patients: report on three cases using an intraoperative PET-CT specimen imager. Urology 182, e257–e261 (2023).
Di Giorgio, A. et al. Long-term outcomes of PSMA PET/CT-guided radiotherapy in biochemical failure patients post-radical prostatectomy: a 5-year follow-up analysis. Eur. J. Nucl. Med. Mol. Imaging 52, 3720–3729 (2025).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05555017 (2023).
Zilli, T. et al. The multicenter, randomized, phase 2 PEACE V-STORM trial: defining the best salvage treatment for oligorecurrent nodal prostate cancer metastases. Eur. Urol. Focus 7, 241–244 (2021).
Knipper, S. et al. Cohort study of oligorecurrent prostate cancer patients: oncological outcomes of patients treated with salvage lymph node dissection via prostate-specific membrane antigen–radioguided surgery. Eur. Urol. 83, 62–69 (2023).
Autio, K. A. et al. Safety and efficacy of BIND-014, a docetaxel nanoparticle targeting prostate-specific membrane antigen for patients with metastatic castration-resistant prostate cancer: a phase 2 clinical trial. JAMA Oncol. 4, 1344–1351 (2018).
Michalska, M. et al. In vitro and in vivo effects of a recombinant anti-PSMA immunotoxin in combination with docetaxel against prostate cancer. Oncotarget 7, 22531–22542 (2016).
Machulkin, A. E. et al. PSMA-targeted small-molecule docetaxel conjugate: synthesis and preclinical evaluation. Eur. J. Med. Chem. 227, 113936 (2022).
Alzubi, J. et al. PSMA-Directed CAR T cells combined with low-dose docetaxel treatment induce tumor regression in a prostate cancer xenograft model. Mol. Ther. Oncolytics 18, 226–235 (2020).
Lim, E. A. et al. Phase 1 study of safety and preliminary clinical activity of JNJ-63898081, a PSMA and CD3 bispecific antibody, for metastatic castration-resistant prostate cancer. Clin. Genitourin. Cancer 21, 366–375 (2023).
Burnell, S. E. A. et al. STEAP2 knockdown reduces the invasive potential of prostate cancer cells. Sci. Rep. 8, 6252 (2018).
Lillard, J. W., Moses, K. A., Mahal, B. A. & George, D. J. Racial disparities in Black men with prostate cancer: a literature review. Cancer 128, 3787–3795 (2022).
Roehrborn, C. G. & Black, L. K. The economic burden of prostate cancer. BJU Int. 108, 806–813 (2011).
Alonso, J. et al. Economic evaluations of radioembolization with yttrium-90 microspheres in liver metastases of colorectal cancer: a systematic review. BMC Gastroenterol. 23, 181 (2023).
Shinto, A., Kamaleshwaran, K., Shibu, D., Vyshak, K. & Antony, J. Empiric therapy with low-dose I-131 in differentiated cancer thyroid: what is the magic number? World J. Nucl. Med. 12, 61–64 (2013).
Olsen, T. A., Filson, C. P., Richards, T. B., Ekwueme, D. U. & Howard, D. H. The cost of metastatic prostate cancer in the United States. Urol. Pract. 10, 41–47 (2023).
Rathke, H. et al. Dosimetry estimate and initial clinical experience with 90Y-PSMA-617. J. Nucl. Med. 60, 806–811 (2019).
International Atomic Energy Agency. Therapeutic Radionuclide Generators: 90Sr/90Yand 188W/188Re Generators (IAEA, 2009).
Kurth, J., Krause, B. J., Hakenberg, O., Schwarzenboeck, S. & Heuschkel, M. [90Y]Y-PSMA-617 for the treatment of metastatic castration-resistant prostate cancer — post-therapeutic kidney and bone marrow dosimetry for individualized therapy. J. Nucl. Med. 61, 190 (2020).
Sobral, M. C., Mota, S. I., Oliveira, P. J., Urbano, A. M. & Paulo, A. Two targets, one mission: heterobivalent metal-based radiopharmaceuticals for prostate cancer imaging and therapy. ChemMedChem 20, e202500128 (2025).
de Klerk, J. M. H. et al. Dose escalation study of rhenium-186 hydroxyethylidene diphosphonate in patients with metastatic prostate cancer. Eur. J. Nucl. Med. 21, 1114–1120 (1994).
Liepe, K., Kropp, J., Runge, R. & Kotzerke, J. Therapeutic efficiency of rhenium-188-HEDP in human prostate cancer skeletal metastases. Br. J. Cancer 89, 625–629 (2003).
Evans-Axelsson, S., Timmermand, O. V., Bjartell, A., Strand, S.-E. & Elgqvist, J. Radioimmunotherapy for prostate cancer — current status and future possibilities. Semin. Nucl. Med. 46, 165–179 (2016).
Lepareur, N. et al. Rhenium-188 labeled radiopharmaceuticals: current clinical applications in oncology and promising perspectives. Front. Med. 6, 132 (2019).
Cardinale, J. et al. PSMA-GCK01: a generator-based 99mTc/188Re theranostic ligand for the prostate-specific membrane antigen. J. Nucl. Med. 64, 1069–1075 (2023).
Trujillo, B., Wu, A., Wetterskog, D. & Attard, G. Blood-based liquid biopsies for prostate cancer: clinical opportunities and challenges. Br. J. Cancer 127, 1394–1402 (2022).
Danila, D. C. et al. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin. Cancer Res. 13, 7053–7058 (2007).
Liu, H., Gao, Y., Vafaei, S., Gu, X. & Zhong, X. The prognostic value of plasma cell-free DNA concentration in the prostate cancer: a systematic review and meta-analysis. Front. Oncol. 11, 599602 (2021).
Vandekerkhove, G. et al. Circulating tumor DNA abundance and potential utility in de novo metastatic prostate cancer. Eur. Urol. 75, 667–675 (2019).
De Giorgi, U. et al. Circulating androgen receptor gene amplification and resistance to 177Lu-PSMA-617 in metastatic castration-resistant prostate cancer: results of a Phase 2 trial. Br. J. Cancer 125, 1226–1232 (2021).
Nimir, M. et al. Detection of AR-V7 in liquid biopsies of castrate resistant prostate cancer patients: a comparison of AR-V7 analysis in circulating tumor cells, circulating tumor RNA and exosomes. Cells 8, 688 (2019).
Quist, S. W., Paulissen, J. H. J., Wyndaele, D. N. J., Nagarajah, J. & Freriks, R. D. Costs of radium-223 and the pharmacy preparation 177Lu-PSMA-I&T for metastatic castration-resistant prostate cancer in Dutch hospitals. J. Med. Econ. 26, 366–375 (2023).
Mehrens, D. et al. Cost-effectiveness analysis of 177Lu-PSMA-617 radioligand therapy in metastatic castration-resistant prostate cancer. J. Natl Compr. Cancer Netw. 21, 43–50.e2 (2023).
Vogel, W. V., van der Marck, S. C. & Versleijen, M. W. J. Challenges and future options for the production of lutetium-177. Eur. J. Nucl. Med. Mol. Imaging 48, 2329–2335 (2021).
Fanti, S. et al. EAU-EANM consensus statements on the role of prostate-specific membrane antigen positron emission tomography/computed tomography in patients with prostate cancer and with respect to [177Lu]Lu-PSMA radioligand therapy. Eur. Urol. Oncol. 5, 530–536 (2022).
Song, R., Jeet, V., Sharma, R., Hoyle, M. & Parkinson, B. Cost-effectiveness analysis of Prostate-Specific Membrane Antigen (PSMA) Positron Emission Tomography/Computed Tomography (PET/CT) for the primary staging of prostate cancer in Australia. Pharmacoeconomics 40, 807–821 (2022).
Ostuni, E. & Taylor, M. R. G. Commercial and business aspects of alpha radioligand therapeutics. Front. Med. 9, 1070497 (2022).
National Institute for Health and Care Excellence. Lutetium-177 Vipivotide Tetraxetan for Treating PSMA-Positive Hormone-Relapsed Metastatic Prostate Cancer after 2 or More Treatments (NICE, 2023).
Thevenot, L. I. & Jacobson, G. M. ASTRO Letter to FDA re Pluvicto 3 14 2023__signed (ASTRO, 2023).
Novartis. Novartis expands production of Pluvicto with addition of its largest and most advanced radioligand therapy manufacturing facility in Indianapolis. Novartis https://www.novartis.com/news/media-releases/novartis-expands-production-pluvictotm-addition-its-largest-and-most-advanced-radioligand-therapy-manufacturing-facility-indianapolis (2024).
Bessette, Z. FDA approves Lutetium-177-PSMA-617 production at New Jersey facility. GU Oncology Now https://www.guoncologynow.com/post/fda-approves-lutetium-177-psma-617-production-at-new-jersey-facility (2023).
Flegar, L. et al. Adoption of Lutetium-177 PSMA radioligand therapy for metastatic castration resistant prostate cancer: a total population analysis in Germany from 2016 to 2020. Eur. J. Nucl. Med. Mol. Imaging 50, 2188–2195 (2023).
Genesiscare. PSMA PET-CT scan. Genesiscare https://www.genesiscare.com/uk/diagnostics/imaging-scans/pet-ct-scan/68gallium-psma-pet-ct-scan.%20Accessed%207th%20June,%202025 (2024).
Africa Infoline. PET CT scan cost in African cities. Africa Infoline https://africainfoline.com/listing/scans/africa/pet-ct-scan-cost-in-african-cities-find-best-hospital-and-book-appointment (2025).
Pramesh, C. S. et al. Priorities for cancer research in low- and middle-income countries: a global perspective. Nat. Med. 28, 649–657 (2022).
Di, M. et al. Costs of care during chimeric antigen receptor T-cell therapy in relapsed or refractory B-cell lymphomas. JNCI Cancer Spectr. 8, pkae059 (2024).
Wang, J. et al. Pretherapeutic 68Ga-PSMA-617 PET may indicate the dosimetry of 177Lu-PSMA-617 and 177Lu-EB-PSMA-617 in main organs and tumor lesions. Clin. Nucl. Med. 44, 431–438 (2019).
Yadav, M. P. et al. Post-therapeutic dosimetry of 177Lu-DKFZ-PSMA-617 in the treatment of patients with metastatic castration-resistant prostate cancer. Nucl. Med. Commun. 38, 91–98 (2017).
Rosar, F. et al. Comparison of different methods for post-therapeutic dosimetry in [177Lu]Lu-PSMA-617 radioligand therapy. EJNMMI Phys. 8, 40 (2021).
Society of Nuclear Medicine and Molecular Imaging. 177Lu PSMA-617 therapy (Pluvicto). SNMMI https://snmmi.org/AM/Patients/Procedures/177Lu-PSMA-617-Therapy-Pluvicto.aspx (2022).
Muniz, M. et al. Salivary toxicity from PSMA-targeted radiopharmaceuticals: what we have learned and where we are going. Cancer Treat. Rev. 127, 102748 (2024).
Emmett, L. et al. ENZA-p trial protocol: a randomized phase II trial using prostate-specific membrane antigen as a therapeutic target and prognostic indicator in men with metastatic castration-resistant prostate cancer treated with enzalutamide (ANZUP 1901). BJU Int. 128, 642–651 (2021).
Herrmann, K. et al. Dosimetry of 177Lu-PSMA-617 for the treatment of metastatic castration-resistant prostate cancer: results from the VISION trial sub-study. J. Clin. Oncol. 40, 97–97 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03454750 (2025).
Maffey-Steffan, J. et al. The 68Ga/177Lu-theragnostic concept in PSMA-targeting of metastatic castration–resistant prostate cancer: impact of post-therapeutic whole-body scintigraphy in the follow-up. Eur. J. Nucl. Med. Mol. Imaging 47, 695–712 (2020).
Alsadi, R., Djekidel, M., Bouhali, O. & Doherty, J. O. Towards routine clinical use of dosimetry in [177Lu]Lu-PSMA prostate cancer radionuclide therapy: current efforts and future perspectives. Front. Phys. 10, 940677 (2022).
Author information
Authors and Affiliations
Contributions
S.A., J.R.G. and Y.W. researched data for the article. All authors substantially to discussion of the content. S.A., J.R.G., Y.W., S.W., A.H., T.S.Y. and G.J.R.C. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M.S.H. acknowledges philanthropic/government grant support from the Prostate Cancer Foundation (PCF), including CANICA Oslo Norway, Peter MacCallum Foundation and NHMRC Investigator Grant; other funding in the past 10 years from Movember/Medical Research Future Fund (MRFF), US Department of Defense and the Prostate Cancer Foundation of Australia (PCFA); research grant support (to Peter MacCallum Cancer Centre) from Novartis, ANSTO, Bayer, Isotopia and MIM; and consulting fees for lectures or advisory boards from Janssen and MSD in the past 2 years and AstraZeneca, Astellas and Mundipharma in the past 5 years. T.S.Y. receives research funding support from AbbVie. G.J.R.C. receives research support from Theragnostics, NanoMab Technology (UK) and Siemens Healthineers; provides consultancy and advisory board support to Serac Healthcare, Amgen, Telix Pharmaceuticals, Blue Earth Diagnostics, NanoMab Technology (UK), GE Healthcare and Full Life Technology; and receives lecture fees from Telix Pharmaceuticals and Curium. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks Fransesco Ceci, Tobias Maurer, Mike Sathekge, Ken Herrmann and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Adeleke, S., Galante, J.R., Wang, Y. et al. Emerging evidence for sequencing and combining PSMA-based therapies in prostate cancer. Nat Rev Urol (2025). https://doi.org/10.1038/s41585-025-01107-6
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41585-025-01107-6