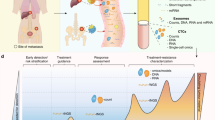
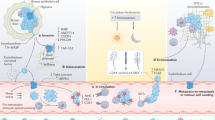
Abstract
Prostate cancer is characterized by multifocality, inter- and intra-patient tumour heterogeneity, and differences in risk of progression to metastatic disease, castration resistance and lethality, which can make prognosis challenging. Consequently, sampling methods that provide accurate insight into disease phenotype to facilitate risk-stratification of patients are crucial. The variable biology of prostate cancer seems to be recapitulated in the phenotypic heterogeneity of circulating tumour cells (CTCs). CTC sampling offers a liquid biopsy method to achieve minimally invasive longitudinal sampling for disease monitoring. CTC analysis has also offered a crucial insight into aggressive phenotypes, disease metastasis and treatment response, particularly in clinical trials. The clinical use of CTC count for prognosis in advanced prostate cancer has been approved by the FDA, but is not routinely used clinically, as these cells are technically challenging to isolate and analyse. However, methodological advances continue to improve CTC enrichment and profiling. Understanding the clinical utility of CTCs and future innovations is crucial to incorporating CTCs into the clinical management of prostate cancer.
Key points
-
Circulating tumour cells (CTCs) can be detected in blood from subsets of patients with prostate cancer by using antigen-dependent or -independent methods of enrichment.
-
New label-free enrichment strategies are renewing interest in CTCs after advances in next-generation sequencing had previously shifted the focus of liquid biopsy to cell-free DNA (cfDNA).
-
Molecular profiling of prostate cancer CTCs might offer a stratification tool for selecting patients for systemic therapy.
-
CTCs provide insight into the clinical and biological heterogeneity of prostate cancer, potentially offering markers of prognosis and/or treatment response.
-
Further study is needed on the role of CTCs in localized prostate cancer, requiring increased sensitivity and specificity of enrichment methods.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Erickson, A. et al. Spatially resolved clonal copy number alterations in benign and malignant tissue. Nature 608, 360–367 (2022).
Haffner, M. C. et al. Genomic and phenotypic heterogeneity in prostate cancer. Nat. Rev. Urol. 18, 79–92 (2021).
Baca, S. C. et al. Punctuated evolution of prostate cancer genomes. Cell 153, 666–677 (2013).
Delanoy, N. et al. Clinical progression is associated with poor prognosis whatever the treatment line in metastatic castration resistant prostate cancer: the CATS international database. Eur. J. Cancer 125, 153–163 (2020).
Ramos-Montoya, A. et al. HES6 drives a critical AR transcriptional programme to induce castration-resistant prostate cancer through activation of an E2F1-mediated cell cycle network. EMBO Mol. Med. 6, 651–661 (2014).
Lamb, A. D., Massie, C. E. & Neal, D. E. The transcriptional programme of the androgen receptor (AR) in prostate cancer. BJU Int. 113, 358–366 (2014).
Ross-Adams, H. et al. Integration of copy number and transcriptomics provides risk stratification in prostate cancer: a discovery and validation cohort study. EBioMedicine 2, 1133–1144 (2015).
Dijkstra, S., Hamid, A. R. A. H., Leyten, G. H. J. M. & Schalken, J. A. Personalized management in low-risk prostate cancer: role of biomarkers. Prostate Cancer 2012, 1–7 (2012).
Singhal, U. et al. Integrative multi-region molecular profiling of primary prostate cancer in men with synchronous lymph node metastasis. Nat. Commun. 15, 1–9 (2024).
Cooper, C. S. et al. Analysis of the genetic phylogeny of multifocal prostate cancer identifies multiple independent clonal expansions in neoplastic and morphologically normal prostate tissue. Nat. Genet. 47, 367–372 (2015).
Erickson, A. et al. A systematic review of prostate cancer heterogeneity: understanding the clonal ancestry of multifocal disease. Eur. Urol. Oncol. 4, 358–369 (2021).
Marklund, M. et al. Spatio-temporal analysis of prostate tumors in situ suggests pre-existence of treatment-resistant clones. Nat. Commun. 13, 5475 (2022).
Miller, M. C., Doyle, G. V. & Terstappen, L. W. M. M. Significance of circulating tumor cells detected by the CellSearch system in patients with metastatic breast colorectal and prostate cancer. J. Oncol. 2010, 1–8 (2010).
Cristofanilli, M. et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 351, 781–791 (2004).
Fonseca, N. M. et al. Prediction of plasma ctDNA fraction and prognostic implications of liquid biopsy in advanced prostate cancer. Nat. Commun. 15, 1828 (2024).
Tolmeijer, S. H. et al. Early on-treatment changes in circulating tumor DNA fraction and response to enzalutamide or abiraterone in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 29, 2835–2844 (2023).
Tukachinsky, H. et al. Genomic analysis of circulating tumor DNA in 3,334 patients with advanced prostate cancer identifies targetable BRCA alterations and AR resistance mechanisms. Clin. Cancer Res. 27, 3094–3105 (2021).
Helzer, K. T. et al. Fragmentomic analysis of circulating tumor DNA-targeted cancer panels. Ann. Oncol. 34, 813–825 (2023).
Conteduca, V. et al. Circulating androgen receptor for prognosis and treatment selection in prostate cancer. Eur. Urol. Oncol. 4, 740–744 (2021).
Wyatt, A. W. et al. Concordance of circulating tumor DNA and matched metastatic tissue biopsy in prostate cancer. J. Natl Cancer Inst. 109, djx118 (2017).
Beltran, H. et al. Circulating tumor DNA profile recognizes transformation to castration-resistant neuroendocrine prostate cancer. J. Clin. Invest. 130, 1653–1668 (2020).
Herberts, C. et al. Deep whole-genome ctDNA chronology of treatment-resistant prostate cancer. Nature 608, 199–208 (2022).
Garofoli, M. et al. Circulating tumor DNA: a new research frontier in urological oncology from localized to metastatic disease. Eur. Urol. Oncol. 8, 805–817 (2025).
Maia, M. C., Salgia, M. & Pal, S. K. Harnessing cell-free DNA: plasma circulating tumour DNA for liquid biopsy in genitourinary cancers. Nat. Rev. Urol. 17, 271–291 (2020).
Lin, D. et al. Circulating tumor cells: biology and clinical significance. Signal. Transduct. Target. Ther. 6, 404 (2021).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Ring, A., Nguyen-Strauli, B. D., Wicki, A. & Aceto, N. Biology, vulnerabilities and clinical applications of circulating tumour cells. Nat. Rev. Cancer 23, 95–111 (2023).
Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 133, 571–573 (1889).
Akhtar, M., Haider, A., Rashid, S. & Al-Nabet, A. D. M. H. Paget’s “seed and soil” theory of cancer metastasis: an idea whose time has come. Adv. Anat. Pathol. 26, 69–74 (2019).
Heidrich, I., Deitert, B., Werner, S. & Pantel, K. Liquid biopsy for monitoring of tumor dormancy and early detection of disease recurrence in solid tumors. Cancer Metastasis Rev. 42, 161–182 (2023).
Chu, X. et al. Cancer stem cells: advances in knowledge and implications for cancer therapy. Signal. Transduct. Target. Ther. 9, 170 (2024).
Mei, W. et al. The contributions of prostate cancer stem cells in prostate cancer initiation and metastasis. Cancers 11, 434 (2019).
Xu, L. et al. The novel association of circulating tumor cells and circulating megakaryocytes with prostate cancer prognosis. Clin. Cancer Res. 23, 5112–5122 (2017).
Massard, C. et al. Phenotypic and genetic heterogeneity of tumor tissue and circulating tumor cells in patients with metastatic castration-resistant prostate cancer: a report from the PETRUS prospective study. Oncotarget 7, 55069–55082 (2016).
Gupta, S. et al. Whole genomic copy number alterations in circulating tumor cells from men with abiraterone or enzalutamide-resistant metastatic castration-resistant prostate cancer. Clin. Cancer Res. 23, 1346–1357 (2017).
Wu, C., Xu, C., Wang, G., Zhang, D. & Zhao, X. Noninvasive circulating tumor cell and urine cellular XPC (rs2228001, A2815C) and XRCC1 (rs25487, G1196A) polymorphism detection as an effective screening panel for genitourinary system cancers. Transl. Cancer Res. 8, 2803–2812 (2019).
Punnoose, E. A. et al. PTEN loss in circulating tumour cells correlates with PTEN loss in fresh tumour tissue from castration-resistant prostate cancer patients. Br. J. Cancer 113, 1225–1233 (2015).
Pezzi, H. M. et al. Versatile exclusion-based sample preparation platform for integrated rare cell isolation and analyte extraction. Lab. Chip 18, 3446–3458 (2018).
Zheng, Y. et al. Prognostic value of circulating tumor cells in castration resistant prostate cancer: a meta-analysis. Urol. J. 13, 2881–2888 (2016).
Salami, S. S. et al. Circulating tumor cells as a predictor of treatment response in clinically localized prostate cancer. JCO Precis. Oncol. 3, PO.18.00352 (2019).
Al-Hammouri, T. et al. Protocol for a prospective study evaluating circulating tumour cells status to predict radical prostatectomy treatment failure in localised prostate cancer patients (C-ProMeta-1). BMC Cancer 23, 581 (2023).
Lawrence, R., Watters, M., Davies, C. R., Pantel, K. & Lu, Y.-J. Circulating tumour cells for early detection of clinically relevant cancer. Nat. Rev. Clin. Oncol. 20, 487–500 (2023).
Xu, L. et al. Noninvasive detection of clinically significant prostate cancer using circulating tumor cells. J. Urol. 203, 73–82 (2020).
Ren, X., He, X., Xu, C., Han, D. & Cheng, S. Functional tumor targeting nano-systems for reprogramming circulating tumor cells with in situ evaluation on therapeutic efficiency at the single-cell level. Adv. Sci. 9, e2105806 (2022).
Reichert, Z. R. et al. Multigene profiling of circulating tumor cells (CTCs) for prognostic assessment in treatment-naïve metastatic hormone-sensitive prostate cancer (mHSPC). Int. J. Mol. Sci. 23, 4 (2021).
Davies, C. R. et al. The potential of using circulating tumour cells and their gene expression to predict docetaxel response in metastatic prostate cancer. Front. Oncol. 12, 1060864 (2023).
Vandekerkhove, G., Chi, K. N. & Wyatt, A. W. Clinical utility of emerging liquid biomarkers in advanced prostate cancer. Cancer Genet. 228–229, 151–158 (2018).
Kruck, S., Gakis, G. & Stenzl, A. Disseminated and circulating tumor cells for monitoring chemotherapy in urological tumors. Anticancer Res. 31, 2053–2057 (2011).
Cieślikowski, W. A., Antczak, A., Nowicki, M., Zabel, M. & Budna-Tukan, J. Clinical relevance of circulating tumor cells in prostate cancer management. Biomedicines 9, 1179 (2021).
Zhang, T. & Armstrong, A. J. Clinical utility of circulating tumor cells in advanced prostate cancer. Curr. Oncol. Rep. 18, 3 (2016).
Strati, A., Markou, A., Kyriakopoulou, E. & Lianidou, E. Detection and molecular characterization of circulating tumour cells: challenges for the clinical setting. Cancers 15, 2185 (2023).
Templeman, A. et al. Analytical performance of the FDA-cleared Parsortix® PC1 system. J. Circ. Biomark. 12, 26–33 (2023).
Millner, L. M., Linder, M. W. & Valdes, R. Circulating tumor cells: a review of present methods and the need to identify heterogeneous phenotypes. Ann. Clin. Lab. Sci. 43, 295–304 (2013).
Shen, W. et al. Combined immunomagnetic capture coupled with ultrasensitive plasmonic detection of circulating tumor cells in blood. Biomed. Microdevices 20, 99 (2018).
Bitting, R. L. et al. Development of a method to isolate circulating tumor cells using mesenchymal-based capture. Methods 64, 129–136 (2013).
Zhang, T. et al. Development of a novel c-MET-based CTC detection platform. Mol. Cancer Res. 14, 539–547 (2016).
Cha, J., Cho, H., Chung, J.-S., Park, J. S. & Han, K.-H. Effective circulating tumor cell isolation using epithelial and mesenchymal markers in prostate and pancreatic cancer patients. Cancers 15, 2825 (2023).
Vila, A. et al. EGFR-based immunoisolation as a recovery target for low-EpCAM CTC subpopulation. PLoS ONE 11, e0163705 (2016).
Magbanua, M. J. M. et al. Isolation and genomic analysis of circulating tumor cells from castration resistant metastatic prostate cancer. BMC Cancer 12, 78 (2012).
Autebert, J. et al. High purity microfluidic sorting and analysis of circulating tumor cells: towards routine mutation detection. Lab. Chip 15, 2090–2101 (2015).
Glia, A. et al. Herringbone microfluidic probe for multiplexed affinity-capture of prostate circulating tumor cells. Adv. Mater. Technol. 6, 2100053 (2021).
Yin, C. et al. Molecular profiling of pooled circulating tumor cells from prostate cancer patients using a dual-antibody-functionalized microfluidic device. Anal. Chem. 90, 3744–3751 (2018).
Zhao, L. et al. High-purity prostate circulating tumor cell isolation by a polymer nanofiber-embedded microchip for whole exome sequencing. Adv. Mater. 25, 2897–2902 (2013).
Chu, C.-H. et al. Negative enrichment of circulating tumor cells from unmanipulated whole blood with a 3D printed device. Sci. Rep. 11, 20583 (2021).
Huang, Y.-Y. et al. Screening and molecular analysis of single circulating tumor cells using micromagnet array. Sci. Rep. 5, 16047 (2015).
Fachin, F. et al. Monolithic chip for high-throughput blood cell depletion to sort rare circulating tumor cells. Sci. Rep. 7, 10936 (2017).
Mentink, A., Isebia, K. T., Kraan, J., Terstappen, L. W. M. M. & Stevens, M. Measuring antigen expression of cancer cell lines and circulating tumour cells. Sci. Rep. 13, 6051 (2023).
de Wit, S. et al. EpCAMhigh and EpCAMlow circulating tumor cells in metastatic prostate and breast cancer patients. Oncotarget 9, 35705–35716 (2018).
Saxena, K., Subbalakshmi, A. R. & Jolly, M. K. Phenotypic heterogeneity in circulating tumor cells and its prognostic value in metastasis and overall survival. EBioMedicine 46, 4–5 (2019).
Brouwer, A. et al. Evaluation and consequences of heterogeneity in the circulating tumor cell compartment. Oncotarget 7, 48625–48643 (2016).
Jiang, W. et al. Bait-trap chip for accurate and ultrasensitive capture of living circulating tumor cells. Acta Biomater. 162, 226–239 (2023).
Myung, J. H. et al. Dendrimer-based platform for effective capture of tumor cells after TGFβ 1-induced epithelial–mesenchymal transition. Anal. Chem. 91, 8374–8382 (2019).
Witek, M. A. et al. Discrete microfluidics for the isolation of circulating tumor cell subpopulations targeting fibroblast activation protein alpha and epithelial cell adhesion molecule. NPJ Precis. Oncol. 1, 24 (2017).
Chen, J. et al. Feasibility study of expressing epcam + /vimentin + CTC in prostate cancer diagnosis. J. Cancer Res. Clin. Oncol. 149, 8699–8709 (2023).
Hughes, A. D. et al. Microtube device for selectin-mediated capture of viable circulating tumor cells from blood. Clin. Chem. 58, 846–853 (2012).
Deliorman, M. et al. AFM-compatible microfluidic platform for affinity-based capture and nanomechanical characterization of circulating tumor cells. Microsyst. Nanoeng. 6, 20 (2020).
Wang, B. et al. Evaporation-induced rGO coatings for highly sensitive and non-invasive diagnosis of prostate cancer in the PSA gray zone. Adv. Mater. 33, e2103999 (2021).
Gleghorn, J. P. et al. Capture of circulating tumor cells from whole blood of prostate cancer patients using geometrically enhanced differential immunocapture (GEDI) and a prostate-specific antibody. Lab. Chip 10, 27–29 (2010).
Zhang, J., Chen, K. & Fan, Z. H. Circulating tumor cell isolation and analysis. Adv. Clin. Chem. 75, 1–31 (2016).
Sperger, J. M. et al. Expression and therapeutic targeting of TROP-2 in treatment-resistant prostate cancer. Clin. Cancer Res. 29, 2324–2335 (2023).
Gires, O., Pan, M., Schinke, H., Canis, M. & Baeuerle, P. A. Expression and function of epithelial cell adhesion molecule EpCAM: where are we after 40 years? Cancer Metastasis Rev. 39, 969–987 (2020).
Chiu, J.-J. et al. Mechanisms of induction of endothelial cell E-selectin expression by smooth muscle cells and its inhibition by shear stress. Blood 110, 519–528 (2007).
Chang, S. S. Overview of prostate-specific membrane antigen. Rev. Urol. 6 (Suppl. 10), S13–S18 (2004).
Balk, S. P., Ko, Y.-J. & Bubley, G. J. Biology of prostate-specific antigen. J. Clin. Oncol. 21, 383–391 (2003).
Ivaska, J. Vimentin: central hub in EMT induction? Small GTPases 2, 51–53 (2011).
Rajaram, P. et al. Epidermal growth factor receptor: role in human cancer. Indian J. Dental Res. 28, 687 (2017).
Zhang, Y. et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol. Cancer 17, 45 (2018).
Lira, C. B. B., Chu, K., Lee, Y.-C., Hu, M. C.-T. & Lin, S.-H. Expression of the extracellular domain of OB-cadherin as an Fc fusion protein using bicistronic retroviral expression vector. Protein Expr. Purif. 61, 220–226 (2008).
Day, K. C. et al. HER2 and EGFR overexpression support metastatic progression of prostate cancer to bone. Cancer Res. 77, 74–85 (2017).
Shen, M., Liu, S. & Stoyanova, T. The role of Trop2 in prostate cancer: an oncogene, biomarker, and therapeutic target. Am. J. Clin. Exp. Urol. 9, 73–87 (2021).
Saeki, N., Gu, J., Yoshida, T. & Wu, X. Prostate stem cell antigen: a Jekyll and Hyde molecule? Clin. Cancer Res. 16, 3533–3538 (2010).
Theil, G., Bialek, J., Weiß, C., Lindner, F. & Fornara, P. Strategies for isolating and propagating circulating tumor cells in men with metastatic prostate cancer. Diagnostics 12, 497 (2022).
Hupert, M. L. et al. Arrays of high-aspect ratio microchannels for high-throughput isolation of circulating tumor cells (CTCs). Microsyst. Technol. 20, 1815–1825 (2014).
Awe, J. A., Saranchuk, J., Drachenberg, D. & Mai, S. Filtration-based enrichment of circulating tumor cells from all prostate cancer risk groups. Urol. Oncol. 35, 300–309 (2017).
Mendelaar, P. A. J. et al. Defining the dimensions of circulating tumor cells in a large series of breast, prostate, colon, and bladder cancer patients. Mol. Oncol. 15, 116–125 (2021).
Schmid-Schonbein, G., Shih, Y. & Chien, S. Morphometry of human leukocytes. Blood 56, 866–875 (1980).
Diez-Silva, M., Dao, M., Han, J., Lim, C.-T. & Suresh, S. Shape and biomechanical characteristics of human red blood cells in health and disease. MRS Bull. 35, 382–388 (2010).
Adebayo Awe, J. et al. Three-dimensional telomeric analysis of isolated circulating tumor cells (CTCs) defines CTC subpopulations. Transl. Oncol. 6, 51–65 (2013).
Xu, L. et al. Optimization and evaluation of a novel size based circulating tumor cell isolation system. PLoS ONE 10, e0138032 (2015).
Augustsson, P., Magnusson, C., Nordin, M., Lilja, H. & Laurell, T. Microfluidic, label-free enrichment of prostate cancer cells in blood based on acoustophoresis. Anal. Chem. 84, 7954–7962 (2012).
Di Trapani, M., Manaresi, N. & Medoro, G. DEPArray™ system: an automatic image-based sorter for isolation of pure circulating tumor cells. Cytometry Part. A 93, 1260–1266 (2018).
Wu, M. et al. Circulating tumor cell phenotyping via high-throughput acoustic separation. Small 14, e1801131 (2018).
Liu, J. et al. Circulating tumor cells (CTCs): a unique model of cancer metastases and non-invasive biomarkers of therapeutic response. Front. Genet. 12, 734595 (2021).
Andree, K. C. et al. Toward a real liquid biopsy in metastatic breast and prostate cancer: diagnostic leukapheresis increases CTC yields in a European prospective multicenter study (CTCTrap). Int. J. Cancer 143, 2584–2591 (2018).
Theil, G. et al. In vivo isolation of circulating tumor cells in patients with different stages of prostate cancer. Oncol. Lett. 21, 357 (2021).
Theil, G. et al. The use of a new CellCollector to isolate circulating tumor cells from the blood of patients with different stages of prostate cancer and clinical outcomes — a proof-of-concept study. PLoS ONE 11, e0158354 (2016).
Zapatero, A. et al. Detection and dynamics of circulating tumor cells in patients with high-risk prostate cancer treated with radiotherapy and hormones: a prospective phase II study. Radiat. Oncol. 15, 137 (2020).
Ligthart, S. T. et al. Circulating tumor cells count and morphological features in breast, colorectal and prostate cancer. PLoS ONE 8, e67148 (2013).
Ju, S. et al. Detection of circulating tumor cells: opportunities and challenges. Biomark. Res. 10, 58 (2022).
Pal, S. K. et al. Detection and phenotyping of circulating tumor cells in high-risk localized prostate cancer. Clin. Genitourin. Cancer 13, 130–136 (2015).
Kerr, B. A. et al. CD117+ cells in the circulation are predictive of advanced prostate cancer. Oncotarget 6, 1889–1897 (2015).
Murray, N. P., Reyes, E., Orellana, N., Fuentealba, C. & Dueñas, R. A comparative performance analysis of total PSA, percentage free PSA, PSA velocity, and PSA density versus the detection of primary circulating prostate cells in predicting initial prostate biopsy findings in Chilean men. Biomed. Res. Int. 2014, 1–8 (2014).
Limaye, S. et al. Accurate prostate cancer detection based on enrichment and characterization of prostate cancer specific circulating tumor cells. Cancer Med. 12, 9116–9127 (2023).
de Bono, J. S. et al. Potential applications for circulating tumor cells expressing the insulin-like growth factor-I receptor. Clin. Cancer Res. 13, 3611–3616 (2007).
Pal, S. K. et al. Synaptophysin expression on circulating tumor cells in patients with castration resistant prostate cancer undergoing treatment with abiraterone acetate or enzalutamide. Urol. Oncol. 36, 162.e1–162.e6 (2018).
Saha, S. K., Islam, S. M. R., Kwak, K.-S., Rahman, M. S. & Cho, S.-G. PROM1 and PROM2 expression differentially modulates clinical prognosis of cancer: a multiomics analysis. Cancer Gene Ther. 27, 147–167 (2020).
van Roy, F. & Berx, G. The cell-cell adhesion molecule E-cadherin. Cell. Mol. Life Sci. 65, 3756–3788 (2008).
Gatalica, Z., Stafford, P. & Vranic, S. Alpha-methylacyl-CoA racemase (AMACR) protein is upregulated in early proliferative lesions of the breast irrespective of apocrine differentiation. Hum. Pathol. 129, 40–46 (2022).
Gladson, C. L. & Welch, D. R. New insights into the role of CXCR4 in prostate cancer metastasis. Cancer Biol. Ther. 7, 1849–1851 (2008).
Radu, P. et al. CD34-structure, functions and relationship with cancer stem cells. Medicina 59, 938 (2023).
Harris, K. S. et al. CD117/c-kit defines a prostate CSC-like subpopulation driving progression and TKI resistance. Sci. Rep. 11, 1465 (2021).
Wu, J. & Yu, E. Insulin-like growth factor receptor-1 (IGF-IR) as a target for prostate cancer therapy. Cancer Metastasis Rev. 33, 607–617 (2014).
Wiedenmann, B., Franke, W. W., Kuhn, C., Moll, R. & Gould, V. E. Synaptophysin: a marker protein for neuroendocrine cells and neoplasms. Proc. Natl Acad. Sci. 83, 3500–3504 (1986).
Toss, A., Mu, Z., Fernandez, S. & Cristofanilli, M. CTC enumeration and characterization: moving toward personalized medicine. Ann. Transl. Med. 2, 108 (2014).
Enikeev, D., Morozov, A., Babaevskaya, D., Bazarkin, A. & Malavaud, B. A systematic review of circulating tumor cells clinical application in prostate cancer diagnosis. Cancers 14, 3802 (2022).
Helo, P. et al. Circulating prostate tumor cells detected by reverse transcription-PCR in men with localized or castration-refractory prostate cancer: concordance with CellSearch assay and association with bone metastases and with survival. Clin. Chem. 55, 765–773 (2009).
Skerenova, M., Mikulova, V., Capoun, O., Zima, T. & Tesarova, P. Circulating tumor cells and serum levels of MMP-2, MMP-9 and VEGF as markers of the metastatic process in patients with high risk of metastatic progression. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 161, 272–280 (2017).
Albino, G., Vendittelli, F., Paolillo, C., Zuppi, C. & Capoluongo, E. Potential usefulness of CTC detection in follow up of prostate cancer patients. A preliminary report obtained by using Adnagene platform. Arch. Ital. Urol. Androl. 85, 164–169 (2013).
Todenhöfer, T. et al. Preliminary experience on the use of the Adnatest® system for detection of circulating tumor cells in prostate cancer patients. Anticancer. Res. 32, 3507–3513 (2012).
Sperger, J. M. et al. Prospective evaluation of clinical outcomes using a multiplex liquid biopsy targeting diverse resistance mechanisms in metastatic prostate cancer. J. Clin. Oncol. 39, 2926–2937 (2021).
Thiounn, N. et al. Positive prostate-specific antigen circulating cells detected by reverse transcriptase-polymerase chain reaction does not imply the presence of prostatic micrometastases. Urology 50, 245–250 (1997).
Bos, M. K., Kraan, J., Sleijfer, S., Martens, J. W. M. & Beije, N. Prognostic value of circulating tumor cell characteristics may be biased by their quantity. J. Clin. Oncol. 40, 519–520 (2022).
Xu, C., He, X.-Y., Ren, X.-H., Han, D. & Cheng, S.-X. Detection of mRNAs of ribosomal protein L15 and E-cadherin in living circulating tumor cells at single cell resolution to study tumor heterogeneity. Anal. Chem. 94, 10610–10616 (2022).
Kuske, A. et al. Improved detection of circulating tumor cells in non-metastatic high-risk prostate cancer patients. Sci. Rep. 6, 1–9 (2016).
Chen, S. et al. In vivo detection of circulating tumor cells in high-risk non-metastatic prostate cancer patients undergoing radiotherapy. Cancers 11, 933 (2019).
Budna-Tukan, J. et al. Analysis of circulating tumor cells in patients with non-metastatic high-risk prostate cancer before and after radiotherapy using three different enumeration assays. Cancers 11, 802 (2019).
Gao, Y. et al. Single-cell sequencing deciphers a convergent evolution of copy number alterations from primary to circulating tumor cells. Genome Res. 27, 1312–1322 (2017).
Dago, A. E. et al. Rapid phenotypic and genomic change in response to therapeutic pressure in prostate cancer inferred by high content analysis of single circulating tumor cells. PLoS ONE 9, e101777 (2014).
Stankiewicz, E. et al. Identification of FBXL4 as a metastasis associated gene in prostate cancer. Sci. Rep. 7, 5124 (2017).
Lohr, J. G. et al. Whole-exome sequencing of circulating tumor cells provides a window into metastatic prostate cancer. Nat. Biotechnol. 32, 479–484 (2014).
Gorges, T. M. et al. Accession of tumor heterogeneity by multiplex transcriptome profiling of single circulating tumor cells. Clin. Chem. 62, 1504–1515 (2016).
Nagaya, N. et al. Prostate-specific membrane antigen in circulating tumor cells is a new poor prognostic marker for castration-resistant prostate cancer. PLoS ONE 15, e0226219 (2020).
Groen, L. et al. Transcriptome profiling of circulating tumor cells to predict clinical outcomes in metastatic castration-resistant prostate cancer. Int. J. Mol. Sci. 24, 9002 (2023).
Heck, M. M. et al. A 2-gene panel derived from prostate cancer-enhanced transcripts in whole blood is prognostic for survival and predicts treatment benefit in metastatic castration-resistant prostate cancer. Prostate 76, 1160–1168 (2016).
Chen, C. et al. Single-cell analysis of circulating tumor cells identifies cumulative expression patterns of EMT-related genes in metastatic prostate cancer. Prostate 73, 813–826 (2013).
McKay, R. R. et al. Phase II multicenter study of enzalutamide in metastatic castration-resistant prostate cancer to identify mechanisms driving resistance. Clin. Cancer Res. 27, 3610–3619 (2021).
Cho, H. et al. Multigene model for predicting metastatic prostate cancer using circulating tumor cells by microfluidic magnetophoresis. Cancer Sci. 112, 859–870 (2021).
Miyamoto, D. T. et al. RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. Science 349, 1351–1356 (2015).
Pal, S. K. et al. Identification of mechanisms of resistance to treatment with abiraterone acetate or enzalutamide in patients with castration-resistant prostate cancer (CRPC). Cancer 124, 1216–1224 (2018).
Singhal, U. et al. Multigene profiling of CTCs in mCRPC identifies a clinically relevant prognostic signature. Mol. Cancer Res. 16, 643–654 (2018).
Beltran, H. et al. The role of lineage plasticity in prostate cancer therapy resistance. Clin. Cancer Res. 25, 6916–6924 (2019).
Armstrong, A. J. et al. Prospective multicenter validation of androgen receptor splice variant 7 and hormone therapy resistance in high-risk castration-resistant prostate cancer: the PROPHECY study. J. Clin. Oncol. 37, 1120–1129 (2019).
Antonarakis, E. S. et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 371, 1028–1038 (2014).
Zhao, S. G. et al. A clinical-grade liquid biomarker detects neuroendocrine differentiation in prostate cancer. J. Clin. Invest. 132, e161858 (2022).
Castellani, G. et al. BRAF mutations in melanoma: biological aspects, therapeutic implications, and circulating biomarkers. Cancers 15, 4026 (2023).
Morrison, G. et al. Non-invasive profiling of advanced prostate cancer via multi-parametric liquid biopsy and radiomic analysis. Int. J. Mol. Sci. 23, 2571 (2022).
Hodara, E. et al. Multiparametric liquid biopsy analysis in metastatic prostate cancer. JCI Insight 4, e125529 (2019).
Malihi, P. D. et al. Single-cell circulating tumor cell analysis reveals genomic instability as a distinctive feature of aggressive prostate cancer. Clin. Cancer Res. 26, 4143–4153 (2020).
Stover, E. H. et al. Implementation of a prostate cancer-specific targeted sequencing panel for credentialing of patient-derived cell lines and genomic characterization of patient samples. Prostate 82, 584–597 (2022).
Cho, K. S., Oh, H. Y., Lee, E. J. & Hong, S. J. Identification of enhancer of Zeste Homolog 2 expression in peripheral circulating tumor cells in metastatic prostate cancer patients: a preliminary study. Yonsei Med. J. 48, 1009 (2007).
Gjyrezi, A. et al. Androgen receptor variant shows heterogeneous expression in prostate cancer according to differentiation stage. Commun. Biol. 4, 785 (2021).
Reyes, E. E. et al. Molecular analysis of CD133-positive circulating tumor cells from patients with metastatic castration-resistant prostate cancer. J. Transl. Sci. 1, 4 (2015).
Gorges, T. M. et al. Heterogeneous PSMA expression on circulating tumor cells — a potential basis for stratification and monitoring of PSMA-directed therapies in prostate cancer. Oncotarget 7, 34930–34941 (2016).
Chen, J. et al. Metabolic reprogramming-based characterization of circulating tumor cells in prostate cancer. J. Exp. Clin. Cancer Res. 37, 127 (2018).
Gao, D. et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 159, 176–187 (2014).
Mout, L. et al. Generating human prostate cancer organoids from leukapheresis enriched circulating tumour cells. Eur. J. Cancer 150, 179–189 (2021).
Koinis, F. et al. Prognostic role of circulating tumor cells in patients with metastatic castration-resistant prostate cancer receiving cabazitaxel: a prospective biomarker study. Cancers 15, 4511 (2023).
Thalgott, M. et al. Circulating tumor cells versus objective response assessment predicting survival in metastatic castration-resistant prostate cancer patients treated with docetaxel chemotherapy. J. Cancer Res. Clin. Oncol. 141, 1457–1464 (2015).
Gu, T., Li, J., Chen, T., Zhu, Q. & Ding, J. Circulating tumor cell quantification during abiraterone plus prednisone therapy may estimate survival in metastatic castration-resistant prostate cancer patients. Int. Urol. Nephrol. 55, 883–892 (2023).
de Jong, A. C. et al. Liquid biopsies for early response evaluation of radium-223 in metastatic prostate cancer. JCO Precis. Oncol. 7, e2300156 (2023).
Di Lorenzo, G. et al. Assessment of total, PTEN–, and AR-V7+ circulating tumor cell count by flow cytometry in patients with metastatic castration-resistant prostate cancer receiving enzalutamide. Clin. Genitourin. Cancer 19, e286–e298 (2021).
Miyamoto, D. T. et al. An RNA-based digital circulating tumor cell signature is predictive of drug response and early dissemination in prostate cancer. Cancer Discov. 8, 288–303 (2018).
Carles, J. et al. Circulating tumor cells as a biomarker of survival and response to radium-223 therapy: experience in a cohort of patients with metastatic castration-resistant prostate cancer. Clin. Genitourin. Cancer 16, e1133–e1139 (2018).
Hirano, H. et al. Bone scan index (BSI) scoring by using bone scintigraphy and circulating tumor cells (CTCs): predictive factors for enzalutamide effectiveness in patients with castration-resistant prostate cancer and bone metastases. Sci. Rep. 13, 8704 (2023).
Goodman, O. B. et al. Circulating tumor cells as a predictive biomarker in patients with hormone-sensitive prostate cancer. Clin. Genitourin. Cancer 9, 31–38 (2011).
Goldkorn, A. et al. Baseline circulating tumor cell count as a prognostic marker of PSA response and disease progression in metastatic castrate-sensitive prostate cancer (SWOG S1216). Clin. Cancer Res. 27, 1967–1973 (2021).
Yang, G. et al. Clinical significance of mesenchymal circulating tumor cells in patients with oligometastatic hormone-sensitive prostate cancer who underwent cytoreductive radical prostatectomy. Front. Oncol. 11, 812549 (2021).
Ried, K., Eng, P. & Sali, A. Screening for circulating tumour cells allows early detection of cancer and monitoring of treatment effectiveness: an observational study. Asian Pac. J. Cancer Prev. 18, 2275 (2017).
Murray, N. P. et al. Prediction model for early biochemical recurrence after radical prostatectomy based on the Cancer of the Prostate Risk Assessment score and the presence of secondary circulating prostate cells. BJU Int. 118, 556–562 (2016).
Meyer, C. P. et al. Limited prognostic value of preoperative circulating tumor cells for early biochemical recurrence in patients with localized prostate cancer. Urol. Oncol. 34, 235.e11–235.e16 (2016).
Murray, N. P. et al. Head-to-head comparison of the Montreal nomogram with the detection of primary malignant circulating prostate cells to predict prostate cancer at initial biopsy in Chilean men with suspicion of prostate cancer. Urol. Oncol. 33, 203.e19–203.e25 (2015).
Lilja, H. & Scher, H. I. Detection of androgen receptor mutations in circulating tumor cells: highlights of the long road to clinical qualification. Clin. Chem. 56, 1375–1377 (2010).
Kanayama, M., Lu, C., Luo, J. & Antonarakis, E. S. AR splicing variants and resistance to AR targeting agents. Cancers 13, 2563 (2021).
Jiang, Y., Palma, J. F., Agus, D. B., Wang, Y. & Gross, M. E. Detection of androgen receptor mutations in circulating tumor cells in castration-resistant prostate cancer. Clin. Chem. 56, 1492–1495 (2010).
Ma, Y. et al. Droplet digital PCR based androgen receptor variant 7 (AR-V7) detection from prostate cancer patient blood biopsies. Int. J. Mol. Sci. 2016 17, 1264 (2016).
Lu, D. et al. Development of an immunofluorescent AR-V7 circulating tumor cell assay — a blood-based test for men with metastatic prostate cancer. J. Circ. Biomark. 9, 13–19 (2020).
Stuopelyte, K. et al. Analysis of AR-FL and AR-V1 in whole blood of patients with castration resistant prostate cancer as a tool for predicting response to abiraterone acetate. J. Urol. 204, 71–78 (2020).
Scher, H. I. et al. Assessment of the validity of nuclear-localized androgen receptor splice variant 7 in circulating tumor cells as a predictive biomarker for castration-resistant prostate cancer. JAMA Oncol. 4, 1179 (2018).
Antonarakis, E. S. et al. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. 1, 582 (2015).
Antonarakis, E. S. et al. Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J. Clin. Oncol. 35, 2149–2156 (2017).
Erb, H. H. H. et al. AR-V7 protein expression in circulating tumour cells is not predictive of treatment response in mCRPC. Urol. Int. 104, 253–262 (2020).
Okegawa, T. et al. AR-V7 in circulating tumor cells cluster as a predictive biomarker of abiraterone acetate and enzalutamide treatment in castration-resistant prostate cancer patients. Prostate 78, 576–582 (2018).
Hille, C. et al. Detection of androgen receptor variant 7 (ARV7) mRNA levels in EpCAM-enriched CTC fractions for monitoring response to androgen targeting therapies in prostate cancer. Cells 8, 1067 (2019).
Steinestel, J. et al. Detecting predictive androgen receptor modifications in circulating prostate cancer cells. Oncotarget 10, 4213–4223 (2019).
Hench, I. B. et al. Analysis of AR/ARV7 expression in isolated circulating tumor cells of patients with metastatic castration-resistant prostate cancer (SAKK 08/14 IMPROVE trial). Cancers 11, 1099 (2019).
Sieuwerts, A. M. et al. An in-depth evaluation of the validity and logistics surrounding the testing of AR-V7 mRNA expression in circulating tumor cells. J. Mol. Diagn. 20, 316–325 (2018).
Taplin, M.-E. et al. Androgen receptor modulation optimized for response — splice variant: a phase 3, randomized trial of galeterone versus enzalutamide in androgen receptor splice variant-7-expressing metastatic castration-resistant prostate cancer. Eur. Urol. 76, 843–851 (2019).
Bernemann, C. et al. Expression of AR-V7 in circulating tumour cells does not preclude response to next generation androgen deprivation therapy in patients with castration resistant prostate cancer. Eur. Urol. 71, 1–3 (2017).
To, S. Q. et al. Expression of androgen receptor splice variant 7 or 9 in whole blood does not predict response to androgen-axis-targeting agents in metastatic castration-resistant prostate cancer. Eur. Urol. 73, 818–821 (2018).
Scher, H. I. et al. Association of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate cancer. JAMA Oncol. 2, 1441–1449 (2016).
Wang, S. et al. The association of AR-V7 with resistance to abiraterone in metastatic castration-resistant prostate cancer. J. Mens. Health 18, 061 (2022).
Sepe, P. et al. Could circulating tumor cells and ARV7 detection improve clinical decisions in metastatic castration-resistant prostate cancer? The Istituto Nazionale dei Tumori (INT) Experience. Cancers 11, 980 (2019).
Qu, F. et al. Association of AR-V7 and prostate-specific antigen RNA levels in blood with efficacy of abiraterone acetate and enzalutamide treatment in men with prostate cancer. Clin. Cancer Res. 23, 726–734 (2017).
Carles, J. et al. Radium-223 for patients with metastatic castration-resistant prostate cancer with asymptomatic bone metastases progressing on first-line abiraterone acetate or enzalutamide: a single-arm phase II trial. Eur. J. Cancer 173, 317–326 (2022).
Scher, H. I. et al. Nuclear-specific AR-V7 protein localization is necessary to guide treatment selection in metastatic castration-resistant prostate cancer. Eur. Urol. 71, 874–882 (2017).
Graf, R. P. et al. Clinical utility of the nuclear-localized AR-V7 biomarker in circulating tumor cells in improving physician treatment choice in castration-resistant prostate cancer. Eur. Urol. 77, 170–177 (2020).
Montgomery, R. B. & Plymate, S. R. AR-V7 protein in circulating tumor cells — the decider for therapy? JAMA Oncol. 2, 1450 (2016).
Lu, C., Brown, L. C., Antonarakis, E. S., Armstrong, A. J. & Luo, J. Androgen receptor variant-driven prostate cancer II: advances in laboratory investigations. Prostate Cancer Prostatic Dis. 23, 381–397 (2020).
Sprenger, C., Uo, T. & Plymate, S. Androgen receptor splice variant V7 (AR-V7) in circulating tumor cells: a coming of age for AR splice variants? Ann. Oncol. 26, 1805–1807 (2015).
Bazyar, S. et al. Prospective characterization of circulating tumor cells in hormone sensitive oligometastatic prostate cancer patients on a metastasis-directed therapy trial. Int. J. Radiat. Oncol. Biol. Phys. 117, e367–e368 (2023).
Josefsson, A., Damber, J. E. & Welén, K. AR-V7 expression in circulating tumor cells as a potential prognostic marker in metastatic hormone-sensitive prostate cancer. Acta Oncol. 58, 1660–1664 (2019).
Kwan, E. M. et al. Prognostic utility of a whole-blood androgen receptor-based gene signature in metastatic castration-resistant prostate cancer. Eur. Urol. Focus. 7, 63–70 (2021).
Miyamoto, D. T. et al. Androgen receptor signaling in circulating tumor cells as a marker of hormonally responsive prostate cancer. Cancer Discov. 2, 995–1003 (2012).
Tagawa, S. T. et al. Expression of AR-V7 and ARV 567Es in circulating tumor cells correlates with outcomes to taxane therapy in men with metastatic prostate cancer treated in TAXYNERGY. Clin. Cancer Res. 25, 1880–1888 (2019).
Maillet, D. et al. Improved androgen receptor splice variant 7 detection using a highly sensitive assay to predict resistance to abiraterone or enzalutamide in metastatic prostate cancer patients. Eur. Urol. Oncol. 4, 609–617 (2021).
Sharp, A. et al. Clinical utility of circulating tumour cell androgen receptor splice variant-7 status in metastatic castration-resistant prostate cancer. Eur. Urol. 76, 676–685 (2019).
Daniel, M. et al. AR gene rearrangement analysis in liquid biopsies reveals heterogeneity in lethal prostate cancer. Endocr. Relat. Cancer 28, 645–655 (2021).
Acknowledgements
S.M.A. would like to thank the Clarendon Fund in partnership with the St Edmund Hall Kerr-Muir Scholarship, specifically Mr. James Kerr-Muir, for their generous support. T.A. is funded by Cancer Research UK (RCCPDB-Nov23/100013) and supported by the National Institute for Health and Care Research (NIHR). I.G.M., C.M.E. and A.D.L. would like to acknowledge the John Black Charitable Foundation for support. C.M.E. would like to acknowledge support from Rosetrees. A.D.L. is funded by Cancer Research UK (C57899/A25812). Y.J.L. has received funding from Prostate Cancer UK (MA-CT20-011) to investigate the potential of using circulating tumour cells to predict prostate cancer surgical outcome. A.D.L. and R.J.B. are co-CIs of the TRANSLATE prostate biopsy trial funded by HTA (NIHR131233) and are module leads of the QUANTUM Biobank part funded by the John Black Charitable Foundation.
Author information
Authors and Affiliations
Contributions
S.M.A., T.A., D.T.C., N.M.S.R., S.B., C.S., A.D.L. researched data for the article. All authors contributed substantially to discussion of the content. S.M.A., T.A., D.T.C., N.M.S.R., R.B., S.F., W.Y., J.D., S.S., F.C.H., R.J.B., Y.J.L., I.M., C.M.E,. T.M.M., A.D.L. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
S.M.A., C.M.E. and A.D.L. have received support from BioRad through the Celselect grant. T.M.M. is on the advisory boards for Merck, Foundation Medicine, Johnson & Johnson and Pfizer. A.D.L. has received educational support from GlaxoSmithKline, Astellas, Lilly, AstraZeneca and Ipsen. A.D.L. and F.C.H. have paid roles as BJUI Editors. Y.J.L. has received support from ANGLE PLC for his circulating tumour cell study. A.D.L. has received honoraria for reviewing for European Urology and Lancet Oncology. A.D.L. has received consulting fees from AlphaSights. A.D.L. undertakes medicolegal expert witness work related to prostate cancer management in the UK. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abusamra, S.M., Anbarasan, T., Cotton, D.T. et al. Circulating tumour cells as a window into lethality in prostate cancer. Nat Rev Urol (2026). https://doi.org/10.1038/s41585-025-01121-8
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41585-025-01121-8