Abstract
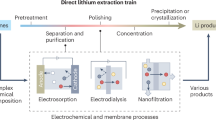
In the quest for environmental sustainability, the rising demand for electric vehicles and renewable energy technologies has substantially increased the need for efficient lithium extraction methods. Traditional lithium production, relying on geographically concentrated hard-rock ores and salar brines, is associated with considerable energy consumption, greenhouse gas emissions, groundwater depletion and land disturbance, thereby posing notable environmental and supply chain challenges. On the other hand, low-quality brines—such as those found in sedimentary waters, geothermal fluids, oilfield-produced waters, seawater and some salar brines and salt lakes—hold large potential owing to their extensive reserves and widespread geographical distribution. However, extracting lithium from these sources presents technical challenges owing to low lithium concentrations and high magnesium-to-lithium ratios. This Review explores the latest advances and continuing challenges in lithium extraction from these demanding yet promising sources, covering a variety of methods, including precipitation, solvent extraction, sorption, membrane-based separation and electrochemical-based separation. Furthermore, we share perspectives on the future development of lithium extraction technologies, framed within the basic principles of separation processes. The aim is to encourage the development of innovative extraction methods capable of making use of the substantial potential of low-quality brines.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wulandari, T., Fawcett, D., Majumder, S. B. & Poinern, G. E. J. Lithium‐based batteries, history, current status, challenges, and future perspectives. Battery Energy 2, 20230030 (2023).
Parlikar, A. et al. High-power electric vehicle charging: low-carbon grid integration pathways with stationary lithium-ion battery systems and renewable generation. Appl. Energy 333, 120541 (2023).
United States Geological Survey (USGS). Mineral commodity summaries 2013–2024. United States Department of the Interior (2013–2024).
International Energy Agency (IEA). Global Critical Minerals Outlook 2024 (2024).
Marjolin, A. Lithium M&A involving assets with resources, H2′21-H1′22. S&P Global https://www.spglobal.com/market-intelligence/en/news-insights/research/lithium-ma-involving-assets-with-resources-h221-to-h122 (2022).
Pehlken, A., Albach, S. & Vogt, T. Is there a resource constraint related to lithium ion batteries in cars? Int. J. Life Cycle Assess. 22, 40–53 (2017).
Bowell, R. J., Lagos, L., de los Hoyos, C. R. & Declercq, J. Classification and characteristics of natural lithium resources. Elements 16, 259–264 (2020).
International Energy Agency (IEA). GHG emissions intensity for lithium by resource type and processing route (2021).
Gutierrez, J. S. et al. Climate change and lithium mining influence flamingo abundance in the lithium triangle. Proc. Biol. Sci. 289, 20212388 (2022).
Baspineiro, C. F., Franco, J. & Flexer, V. Potential water recovery during lithium mining from high salinity brines. Sci. Total Environ. 720, 137523 (2020).
Heubl, B. Lithium firms depleting vital water supplies in Chile, analysis suggests. Institution of Engineering and Technology https://eandt.theiet.org/2019/08/21/lithium-firms-depleting-vital-water-supplies-chile-analysis-suggests (2019).
Hyhne, J. How much water is used to make the world batteries? danwatch https://danwatch.dk/en/undersoegelse/how-much-water-is-used-to-make-the-worlds-batteries/ (2019).
Pure Energy Minerals. Where did that lithium come from? Pure Energy Minerals https://pureenergyminerals.com/technology-overview/.
Castelvecchi, D. Electric cars and batteries: how will the world produce enough? Nature 596, 336–339 (2021).
An, J. W. et al. Recovery of lithium from Uyuni salar brine. Hydrometallurgy 117, 64–70 (2012).
Mousavinezhad, S., Nili, S., Fahimi, A. & Vahidi, E. Environmental impact assessment of direct lithium extraction from brine resources: global warming potential, land use, water consumption, and charting sustainable scenarios. Resour. Conserv. Recycl. 205, 107583 (2024).
Lai, X. R., Xiong, P. & Zhong, H. Extraction of lithium from brines with high Mg/Li ratio by the crystallization-precipitation method. Hydrometallurgy 192, 105252 (2020).
Liu, X. H., Zhong, M. L., Chen, X. Y. & Zhao, Z. W. Separating lithium and magnesium in brine by aluminum-based materials. Hydrometallurgy 176, 73–77 (2018).
Zhou, Z. Y. et al. Recovery of lithium from salt-lake brines using solvent extraction with TBP as extractant and FeCl3 as co-extraction agent. Hydrometallurgy 191, 105244 (2020).
Li, R. J. et al. Selective extraction of lithium ions from salt lake brines using a tributyl phosphate-sodium tetraphenyl boron-phenethyl isobutyrate system. Desalination 555, 116543 (2023).
Chitrakar, R., Kanoh, H., Miyai, Y. & Ooi, K. A new type of manganese oxide (MnO2·0.5H2O) derived from Li1.6Mn1.6O4 and its lithium ion-sieve properties. Chem. Mater. 12, 3151–3157 (2000).
Li, Y. Y., Tang, N., Zhang, L. & Li, J. Fabrication of Fe-doped lithium-aluminum-layered hydroxide chloride with enhanced reusable stability inspired by computational theory and its application in lithium extraction. Colloid. Surf. A 658, 130641 (2023).
Zhang, T. F. et al. Advanced Mg2+/Li+ separation nanofiltration membranes by introducing hydroxypropyltrimethyl ammonium chloride chitosan as a co-monomer. Appl. Surf. Sci. 616, 156434 (2023).
Meng, Q.-W. et al. Enhancing ion selectivity by tuning solvation abilities of covalent-organic-framework membranes. Proc. Natl Acad. Sci. 121, e2316716121 (2024). This insightful work adjusted the length of ether-oxygen chain groups in covalent organic framework pores to modulate the solvation/coordination capacity of membrane pores and investigated their effect on Li+ and Mg2+ transmembrane selectivity.
Liu, C. et al. Lithium extraction from seawater through pulsed electrochemical intercalation. Joule 4, 1459–1469 (2020). An important study developed pulsed-rest and pulse-rest-reverse pulse-rest electrochemical methods with TiO2-coated FePO4 electrodes for Li extraction from seawater. The resulting selectivity for Li and Na reached 1.8 × 104.
Shang, X., Liu, Z. Z., Ji, W. X. & Li, H. B. Synthesis of lithium vanadate/reduced graphene oxide with strong coupling for enhanced capacitive extraction of lithium ions. Sep. Purif. Technol. 262, 118294 (2021).
Munk, L. A. et al. in Rare Earth and Critical Elements in Ore Deposits (eds Verplanck, P. L. & Hitzman, M. W.) 339–365 (Society of Economic Geologists, 2016).
Quintero, C. et al. Development of a co-precipitation process for the preparation of magnesium hydroxide containing lithium carbonate from Li-enriched brines. Hydrometallurgy 198, 105515 (2020).
Yang, S. X., Zhang, F., Ding, H. P., He, P. & Zhou, H. S. Lithium metal extraction from seawater. Joule 2, 1648–1651 (2018). The first article to propose a membrane-based separation method based on hybrid electrolytes for obtaining lithium metal from seawater by electrolysis.
Zhang, Y. et al. A novel precipitant for separating lithium from magnesium in high Mg/Li ratio brine. Hydrometallurgy 187, 125–133 (2019).
Wang, H. Y., Zhong, Y., Du, B. Q., Zhao, Y. J. & Wang, M. Recovery of both magnesium and lithium from high Mg/Li ratio brines using a novel process. Hydrometallurgy 175, 102–108 (2018).
Grant, A. Albemarle Should Build their Magnolia DLE Project. Jade Cove Partners https://www.jadecove.com/research/magnolia (2021).
Goldman Sachs Research. Global Metals & Mining: Direct Lithium Extraction – A potential game changing technology. Goldman Sachs https://www.goldmansachs.com/insights/goldman-sachs-research/direct-lithium-extraction (2023).
Finster, M., Clark, C., Schroeder, J. & Martino, L. Geothermal produced fluids: characteristics, treatment technologies, and management options. Renew. Sustain. Energy Rev. 50, 952–966 (2015).
Seip, A. J. Lithium recovery from hydraulic fracturing flowback and produced water using a manganese-based sorbent. Thesis, Univ. Alberta (2020).
Lee, J. & Chung, E. Lithium recovery by solvent extraction from simulated shale gas produced water – impact of organic compounds. Appl. Geochem. 116, 104571 (2020).
Amakiri, K. T., Ogolo, N. A., Angelis-Dimakis, A. & Albert, O. Physicochemical assessment and treatment of produced water: a case study in Niger delta Nigeria. Pet. Res. 8, 87–95 (2023).
Khatoon, R. et al. Reviewing advanced treatment of hydrocarbon-contaminated oilfield-produced water with recovery of lithium. Sustainability 15, 16016 (2023). This recent review summarized the pretreatment methods on oilfield water bodies before the extraction of lithium.
Yousef, R., Qiblawey, H. & El-Naas, M. H. Adsorption as a process for produced water treatment: a review. Processes 8, 1657 (2020).
Tran, K. T. et al. Recovery of magnesium from Uyuni salar brine as high purity magnesium oxalate. Hydrometallurgy 138, 93–99 (2013). This work used Al-based material to precipitate lithium. The Mg/Li mass ratio in the precipitation was only 0.02.
Liu, D. F., Zhao, Z. W., Xu, W. H., Xiong, J. C. & He, L. H. A closed-loop process for selective lithium recovery from brines via electrochemical and precipitation. Desalination 519, 115302 (2021).
Sun, Q., Chen, H. & Yu, J. G. Investigation on the lithium extraction process with the TBP–FeCl3 solvent system using experimental and DFT methods. Ind. Eng. Chem. Res. 61, 4672–4682 (2022).
Nightingale, E. Jr Phenomenological theory of ion solvation. Effective radii of hydrated ions. J. Phys. Chem. C 63, 1381–1387 (1959).
Oral, I., Tamm, S., Herrmann, C. & Abetz, V. Lithium selectivity of crown ethers: the effect of heteroatoms and cavity size. Sep. Purif. Technol. 294, 121142 (2022). This study researched the ion selectivity for crown ethers by density functional theory, giving a fundamental understanding of crown ethers in lithium extraction.
Li, H. W. et al. Nanofiltration membrane with crown ether as exclusive Li+ transport channels achieving efficient extraction of lithium from salt lake brine. Chem. Eng. J. 438, 135658 (2022).
Onishi, K., Nakamura, T., Nishihama, S. & Yoshizuka, K. Synergistic solvent impregnated resin for adsorptive separation of lithium ion. Ind. Eng. Chem. Res. 49, 6554–6558 (2010).
Su, H. et al. Combining selective extraction and easy stripping of lithium using a ternary synergistic solvent extraction system through regulation of Fe3+ coordination. ACS Sustain. Chem. Eng. 8, 1971–1979 (2020).
Chen, J., Lin, S. & Yu, J. G. High-selective cyclic adsorption and magnetic recovery performance of magnetic lithium-aluminum layered double hydroxides (MLDHs) in extracting Li+ from ultrahigh Mg/Li ratio brines. Sep. Purif. Technol. 255, 117710 (2021).
Zhang, X. S. et al. Porous polyvinyl alcohol/polyacrylamide hydrogels loaded with HTO lithium-ion sieves for highly rapid and efficient Li+ extraction. Desalination 580, 117587 (2024).
Chitrakar, R., Makita, Y., Ooi, K. & Sonoda, A. Lithium recovery from salt lake brine by H2TiO3. Dalton Trans. 43, 8933–8939 (2014).
Wang, J. T. et al. Embedding sulfonated lithium ion-sieves into polyelectrolyte membrane to construct efficient proton conduction pathways. J. Membr. Sci. 501, 109–122 (2016).
Cen, Y. et al. Spinel Li4Mn5O12 as 2.0 V insertion materials for Mg-based hybrid ion batteries. ChemElectroChem 7, 1115–1124 (2020).
Paranthaman, M. P. et al. Recovery of lithium from geothermal brine with lithium–aluminum layered double hydroxide chloride sorbents. Environ. Sci. Technol. 51, 13481–13486 (2017).
Chen, J. et al. Why is aluminum-based lithium adsorbent ineffective in Li+ extraction from sulfate-type brines. AIChE J. 69, e18176 (2023). This work revealed the mechanism for decreased Li+ adsorption performance by using Li/Al-LDH in extracting lithium from sulfate-type brines.
Pan, Y. N., Yu, J. G. & Lin, S. A rational strategy for synchronous extraction of lithium and boron from salt lake brines. Chem. Eng. Sci. 276, 118757 (2023).
Zhang, L. J. et al. Doping engineering of lithium-aluminum layered double hydroxides for high-efficiency lithium extraction from salt lake brines. Nano Res. 17, 1646–1654 (2024).
Chitrakar, R., Kanoh, H., Miyai, Y. & Ooi, K. Recovery of lithium from seawater using manganese oxide adsorbent (H1.6Mn1.6O4) derived from Li1.6Mn1.6O4. Ind. Eng. Chem. Res. 40, 2054–2058 (2001).
Feng, Q., Miyai, Y., Kanoh, H. & Ooi, K. Li+ extraction/insertion with spinel-type lithium manganese oxides: characterization of redox-type and ion-exchange-type sites. Langmuir 8, 1861–1867 (1993).
Liu, S. Q. et al. Reviving the lithium-manganese-based layered oxide cathodes for lithium-ion batteries. Matter 4, 1511–1527 (2021).
Han, H. J., Qu, W., Zhang, Y. L., Lu, H. D. & Zhang, C. L. Enhanced performance of Li+ adsorption for H1.6Mn1.6O4 ion-sieves modified by Co doping and micro array morphology. Ceram. Int. 47, 21777–21784 (2021).
Zhang, G. T. et al. Improved structural stability and adsorption capacity of adsorbent material Li1.6Mn1.6O4 via facile surface fluorination. Colloid. Surf. A 629, 127465 (2021).
Li, J., Zhu, Y., Wang, L. & Cao, C. Lithium titanate epitaxial coating on spinel lithium manganese oxide surface for improving the performance of lithium storage capability. ACS Appl. Mater. Interfaces 6, 18742–18750 (2014).
Wei, S. D., Wei, Y., Chen, T., Liu, C. & Tang, Y. Porous lithium ion sieves nanofibers: general synthesis strategy and highly selective recovery of lithium from brine water. Chem. Eng. J. 379, 122407 (2020).
Zhu, X. L. et al. Study on adsorption extraction process of lithium ion from West Taijinar brine by shaped titanium-based lithium ion sieves. Sep. Purif. Technol. 274, 119099 (2021).
Zhang, J. et al. Bifunctional modification enhances lithium extraction from brine using a titanium-based ion sieve membrane electrode. ACS Appl. Mater. Interfaces 15, 29586–29596 (2023).
Zhang, P. et al. Insight into the synergistic mechanism of Co and N doped titanium-based adsorbents for liquid lithium extraction. Chem. Eng. J. 480, 147631 (2024). A Co and N co-doping strategy for increasing the adsorption sites and improving the kinetics of LTO-type lithium ion sieves.
Chung, K. S. et al. Preparation of ion-sieve type (H)[M0.5Mn1.5]O4 (M=Mg, Zn) and their lithium adsorption properties in seawater. Solid State Phenom. 124, 739–742 (2007).
Panico, D. Development and validation of an electrochemical-thermal model for HIGH ENERGY CELLS and experimental validation. Master’s thesis, Politecnico di Torino (2021).
Hu, F. P., Lin, S., Li, P. & Yu, J. G. Quantitative effects of desorption intensity on structural stability and readsorption performance of lithium/aluminum layered double hydroxides in cyclic Li+ extraction from brines with ultrahigh Mg/Li ratio. Ind. Eng. Chem. Res. 59, 13539–13548 (2020).
Zhang, L. et al. Adsorbents for lithium extraction from salt lake brine with high magnesium/lithium ratio: From structure-performance relationship to industrial applications. Desalination 579, 117480 (2024).
Wang, S. L. et al. Superior lithium adsorption and required magnetic separation behavior of iron-doped lithium ion-sieves. Chem. Eng. J. 332, 160–168 (2018).
Chung, K., Lee, J., Kim, W., Kim, S. & Cho, K. Inorganic adsorbent containing polymeric membrane reservoir for the recovery of lithium from seawater. J. Membr. Sci. 325, 503–508 (2008).
Seip, A. et al. Lithium recovery from hydraulic fracturing flowback and produced water using a selective ion exchange sorbent. Chem. Eng. J. 426, 130713 (2021).
Jang, Y. J. & Chung, E. Lithium adsorptive properties of H2TiO3 adsorbent from shale gas produced water containing organic compounds. Chemosphere 221, 75–80 (2019).
Lucrecia López Steinmetz, R. et al. Northern Puna Plateau-scale survey of Li brine-type deposits in the Andes of NW Argentina. J. Geochem. Explor. 190, 26–38 (2018).
Seader, J. D., Henley, E. J. & Roper, D. K. Separation Process Principles (Wiley, 2006).
Cadotte, J., Forester, R., Kim, M., Petersen, R. & Stocker, T. Nanofiltration membranes broaden the use of membrane separation technology. Desalination 70, 77–88 (1988). This important literature points to the mechanism of membrane separation by nanofiltration, which has been used in many applications.
Wang, X. L., Tsuru, T., Nakao, S. I. & Kimura, S. The electrostatic and steric-hindrance model for the transport of charged solutes through nanofiltration membranes. J. Membr. Sci. 135, 19–32 (1997).
Donnan, F. G. Theorie der membrangleichgewichte und membranpotentiale bei vorhandensein von nicht dialysierenden elektrolyten. Ein beitrag zur physikalisch-chemischen physiologie. Z. Elektrochem. Angew. Phys. Chem. 17, 572–581 (1911).
Peng, Q. et al. Extreme Li-Mg selectivity via precise ion size differentiation of polyamide membrane. Nat. Commun. 15, 2505 (2024). This innovative work regulated the uniform pores for nanofiltration membranes between the hydration diameter of Mg2+ and the Stokes diameter of Li+, resulting an ultrahigh rejection of >99% to Mg2+ and relatively low rejection to Li+.
Liang, H. Q., Guo, Y., Peng, X. S. & Chen, B. L. Light-gated cation-selective transport in metal–organic framework membranes. J. Mater. 8, 11399–11405 (2020).
Guo, Y., Ying, Y., Mao, Y., Peng, X. & Chen, B. Polystyrene sulfonate threaded through a metal–organic framework membrane for fast and selective lithium-ion separation. Angew. Chem. Int. Ed. 55, 15120–15124 (2016).
Pang, X. et al. Enhanced monovalent selectivity of cation exchange membranes via adjustable charge density on functional layers. J. Membr. Sci. 595, 117544 (2020).
Harandi, H. B. & Asadi, A. Transport mechanisms in membranes used for desalination applications. https://doi.org/10.5772/intechopen.1002959 (2023).
Nie, X. Y., Sun, S. Y., Sun, Z., Song, X. F. & Yu, J. G. Ion-fractionation of lithium ions from magnesium ions by electrodialysis using monovalent selective ion-exchange membranes. Desalination 403, 128–135 (2017).
Zhang, B. K., Lu, Y. Y., Li, S. N. & Pan, F. Progress of lithium-ion transport mechanism in solid-state electrolytes. J. Electrochem. 27, 269–277 (2021).
Li, Z. et al. Continuous electrical pumping membrane process for seawater lithium mining. Energy Environ. Sci. 14, 3152–3159 (2021). This important work designed an electrically driven membrane-based separation process by using a solid-state Li+ conductive membrane and enriched lithium concentrations from 0.21 mg l−1 in the Red Sea to 9,013.43 mg l−1.
Li, Z. X. et al. Green lithium: photoelectrochemical extraction. PhotoniX 4, 23 (2023).
Huang, H. et al. Photoelectrochemical lithium extraction. Nano Energy 115, 108683 (2023).
Morita, K., Matsumoto, T. & Hoshino, T. Efficient lithium extraction via electrodialysis using acid-processed lithium-adsorbing lithium lanthanum titanate. Desalination 543, 116117 (2022).
Liu, G., Zhao, Z. W. & He, L. H. Highly selective lithium recovery from high Mg/Li ratio brines. Desalination 474, 114185 (2020).
Zhang, M. H. et al. Research on Li+/Na+ selectivity of NASICON-type solid-state ion conductors by first-principles calculations. Energy Fuels 37, 10663–10672 (2023).
Hong, S. & Elimelech, M. Chemical and physical aspects of natural organic matter (NOM) fouling of nanofiltration membranes. J. Membr. Sci. 132, 159–181 (1997).
Li, Y., Wang, M., Xiang, X., Zhao, Y. J. & Peng, Z. J. Separation performance and fouling analyses of nanofiltration membrane for lithium extraction from salt lake brine. J. Water Process Eng. 54, 104009 (2023).
Shao, S. et al. Biofouling in ultrafiltration process for drinking water treatment and its control by chlorinated-water and pure water backwashing. Sci. Total Environ. 644, 306–314 (2018).
Parsa, N., Moheb, A., Mehrabani-Zeinabad, A. & Masigol, M. A. Recovery of lithium ions from sodium-contaminated lithium bromide solution by using electrodialysis process. Chem. Eng. Res. Des. 98, 81–88 (2015).
Kim, S., Joo, H., Moon, T., Kim, S. H. & Yoon, J. Rapid and selective lithium recovery from desalination brine using an electrochemical system. Environ. Sci. Process. Impacts. 21, 667–676 (2019).
Luo, J. Y., Cui, W. J., He, P. & Xia, Y. Y. Raising the cycling stability of aqueous lithium-ion batteries by eliminating oxygen in the electrolyte. Nat. Chem. 2, 760–765 (2010). A pioneering work that extensively analysed the stability of electrode materials in aqueous solution, which guided the selection of materials for an electrochemical-based separation lithium extraction method.
Gu, J. et al. Multifunctional AlPO4 reconstructed LiMn2O4 surface for electrochemical lithium extraction from brine. J. Energy Chem. 89, 410–421 (2024).
Tan, G., Wan, S., Chen, J. J., Yu, H. Q. & Yu, Y. Reduced lattice constant in Al-doped LiMn2O4 nanoparticles for boosted electrochemical lithium extraction. Adv. Mater. 36, e2310657 (2024).
He, L. et al. New insights into the application of lithium-ion battery materials: selective extraction of lithium from brines via a rocking-chair lithium-ion battery system. Glob. Chall. 2, 1700079 (2018).
Moreau, P., Guyomard, D., Gaubicher, J. & Boucher, F. Structure and stability of sodium intercalated phases in olivine FePO4. Chem. Mater. 22, 4126–4128 (2010).
Yan, G. B., Wang, M. Z., Hill, G. T., Zou, S. Q. & Liu, C. Defining the challenges of Li extraction with olivine host: the roles of competitor and spectator ions. Proc. Natl Acad. Sci. 119, e2200751119 (2022). An important work that analysed the suitability of olivine host materials for extraction from more dilute unconventional water sources and identified related challenges.
Yan, G. B. et al. The role of solid solutions in iron phosphate-based electrodes for selective electrochemical lithium extraction. Nat. Commun. 13, 4579 (2022).
Yan, G. B. et al. Identifying critical features of iron phosphate particle for lithium preference. Nat. Commun. 15, 4859 (2024).
Zhou, J. G., Xiang, S. H., Wang, X. Y., Shin, D. M. & Zhou, H. J. Highly selective lithium extraction from salt lake via carbon-coated lithium vanadium phosphate capacitive electrode. Chem. Eng. J. 482, 148985 (2024).
Jin, W., Hu, M. Q., Sun, Z., Huang, C. H. & Zhao, H. Simultaneous and precise recovery of lithium and boron from salt lake brine by capacitive deionization with oxygen vacancy-rich CoP/Co3O4-graphene aerogel. Chem. Eng. J. 420, 127661 (2021).
Kanoh, H., Ooi, K., Miyai, Y. & Katoh, S. Electrochemical recovery of lithium ions in the aqueous phase. Sep. Sci. Technol. 28, 643–651 (1993). The earliest reported work extracted Li+ from geothermal water based on a Pt–λ-MnO2 system by using an electrochemical-based separation method.
Pasta, M., Battistel, A. & La Mantia, F. Batteries for lithium recovery from brines. Energy Environ. Sci. 5, 9487–9491 (2012).
Kim, S., Kim, J., Kim, S., Lee, J. & Yoon, J. Electrochemical lithium recovery and organic pollutant removal from industrial wastewater of a battery recycling plant. Environ. Sci. Water Res. Technol. 4, 175–182 (2018).
Missoni, L. L., Marchini, F., del Pozo, M. & Calvo, E. J. A LiMn2O4-polypyrrole system for the extraction of LiCl from natural brine. J. Electrochem. Soc. 163, A1898–A1902 (2016).
Bryjak, M., Siekierka, A., Kujawski, J., Smolińska-Kempisty, K. & Kujawski, W. Capacitive deionization for selective extraction of lithium from aqueous solutions. J. Membr. Separ. Technol. 4, 110–115 (2015).
Kim, S., Lee, J., Kim, S., Kim, S. & Yoon, J. Electrochemical lithium recovery with a LiMn2O4-zinc battery system using zinc as a negative electrode. Energy Technol. 6, 340–344 (2018).
Kim, J. S. et al. An electrochemical cell for selective lithium capture from seawater. Environ. Sci. Technol. 49, 9415–9422 (2015).
Zhao, M. Y. et al. Study on lithium extraction from brines based on LiMn2O4/Li1-xMn2O4 by electrochemical method. Electrochim. Acta 252, 350–361 (2017).
Xu, W. H., He, L. H. & Zhao, Z. W. Lithium extraction from high Mg/Li brine via electrochemical intercalation/de-intercalation system using LiMn2O4 materials. Desalination 503, 114935 (2021).
International Battery Metals. A better way: IBAT’s DLE technology vs traditional extraction. https://www.ibatterymetals.com/direct-lithium-extraction/vs-traditional-extraction (2022).
Lewkowicz, J. Can lithium be produced with a lower environmental impact? Dialogue Earth https://dialogochino.net/en/extractive-industries/58865-can-lithium-be-produced-with-lower-environmental-impact-latin-america/ (2022).
Battery Industry. Lithium: Minmetals Salt Lake announces direct lithium extraction breakthrough. Battery Industry https://batteryindustry.tech/lithium-minmetals-salt-lake-announces-direct-lithium-extraction-breakthrough/ (2021).
Chen, X. et al. Spatially separated crystallization for selective lithium extraction from saline water. Nat. Water 1, 808–817 (2023). This work showed an innovative approach allowing quickly evaporated water and selectively extracted lithium from brines by using an array of fibre crystallizers.
Iyer, R. K. & Kelly, J. C. Lithium production in North America: a review. Argonne National Laboratory (ANL) (2023).
Garrett, D. E. Handbook of Lithium and Natural Calcium Chloride: Part 1 – Lithium (Academic Press, 2004).
Gao, F., Zheng, M. P., Nie, Z., Liu, J. H. & Song, S. P. Brine lithium resource in the salt lake and advances in its exploitation. Acta Geosci. Sin. 32, 483–492 (2011).
Gao, C. L., Yu, J. Q., Min, X. Y. & Cheng, A. Y. Hydroclimatic and geothermal controls of lithium brine deposits on the Qinghai-Tibetan Plateau. IOP Conf. Ser. Mater. Sci. Eng. 780, 042062 (2020).
Su, T., Guo, M., Liu, Z. & Li, Q. Comprehensive review of global lithium resources. J. Salt Lake Res. 27, 104–111 (2019).
Liu, C. L. et al. Characteristics, distribution regularity and formation model of brine-type Li deposits in salt lakes in the world. Acta Geol. Sin. 95, 2009–2029 (2021).
Xiang, W., Liang, S. K., Zhou, Z. Y., Qin, W. & Fei, W. Y. Extraction of lithium from salt lake brine containing borate anion and high concentration of magnesium. Hydrometallurgy 166, 9–15 (2016).
Lin, S. N., Zhang, T. A., Pan, X. J. & Zhang, J. J. Eco-friendly extraction of magnesium and lithium from salt lake brine for lithium-ion battery. J. Clean. Prod. 327, 129481 (2021).
Xu, S. S. et al. Extraction of lithium from Chinese salt-lake brines by membranes: design and practice. J. Membr. Sci. 635, 119441 (2021).
Li, Y. L. et al. Origin of lithium-rich salt lakes on the western Kunlun Mountains of the Tibetan Plateau: evidence from hydrogeochemistry and lithium isotopes. Ore Geol. Rev. 155, 105356 (2023).
Dugamin, E. J. M. et al. Groundwater in sedimentary basins as potential lithium resource: a global prospective study. Sci. Rep. 11, 21091 (2021).
Zheng, M. P. & Liu, X. F. Hydrochemistry of salt lakes of the Qinghai-Tibet Plateau, China. Aquat. Geochem. 15, 293–320 (2009).
Zhang, S. X. et al. Solar-driven membrane separation for direct lithium extraction from artificial salt-lake brine. Nat. Commun. 15, 238 (2024). Recently, this study combined a hydrophilic porous membrane driven by capillary force for water transport and an ultrathin ion separation membrane to allow Li+ to pass through and block other multivalent ions, which could direct extraction of lithium from salt-lake brines.
Hamzaoui, A. H., M’Nif, A., Hammi, H. & Rokbani, R. Contribution to the lithium recovery from brine. Desalination 158, 221–224 (2003).
Kaplan, D. Process for the extraction of lithium from Dead Sea solutions. Isr. J. Chem. 1, 115–120 (1965).
Kang, W. W., Zhao, H., Cui, Y., Liu, X. G. & Yang, Y. Z. Construction of novel stable surface ion-imprinted graphene aerogels for efficient and selective extraction of lithium ion. Sep. Purif. Technol. 333, 125946 (2024).
Yang, F. et al. A facile synthesis of hexagonal spinel λ-MnO2 ion-sieves for highly selective Li+ adsorption. Processes 6, 59 (2018).
Li, H. N. et al. Design of photothermal “ion pumps” for achieving energy-efficient, augmented, and durable lithium extraction from seawater. ACS Nano 18, 2434–2445 (2024). This pivotal study designed photothermal ‘ion pumps’, combining a hydrophilic Li+-trapping nanofibrous core and a hydrophobic photothermal shell, leading to substantial enhancement in Li+ trapping rate for extracting lithium.
Liu, L. H. et al. Enhanced lithium-ion adsorption by recyclable lithium manganese oxide-sepiolite composite microsphere from aqueous media: fabrication, structure, and adsorption characteristics. J. Mol. Liq. 380, 121780 (2023).
Chen, J., Lin, S. & Yu, J. Quantitative effects of Fe3O4 nanoparticle content on Li+ adsorption and magnetic recovery performances of magnetic lithium-aluminum layered double hydroxides in ultrahigh Mg/Li ratio brines. J. Hazard. Mater. 388, 122101 (2020). This work proposed the introduction of magnetic particles, Fe3O4, in the synthesis of adsorbent materials to recover the adsorbent materials by the action of a magnetic field.
Su, H. et al. Recovery of lithium from salt lake brine using a mixed ternary solvent extraction system consisting of TBP, FeCl3 and P507. Hydrometallurgy 197, 105487 (2020).
Lu, D. et al. Constructing a selective blocked-nanolayer on nanofiltration membrane via surface-charge inversion for promoting Li+ permselectivity over Mg2+. J. Membr. Sci. 635, 119504 (2021).
Yang, Z. et al. Dual-skin layer nanofiltration membranes for highly selective Li+/Mg2+ separation. J. Membr. Sci. 620, 118862 (2021).
Zhang, H. Z., Xu, Z. L., Ding, H. & Tang, Y. J. Positively charged capillary nanofiltration membrane with high rejection for Mg2+ and Ca2+ and good separation for Mg2+ and Li+. Desalination 420, 158–166 (2017).
Ji, Z. Y. et al. Preliminary study on recovering lithium from high Mg2+/Li+ ratio brines by electrodialysis. Sep. Purif. Technol. 172, 168–177 (2017).
Xu, W. H. et al. Highly selective and efficient lithium extraction from brines by constructing a novel multiple-crack-porous LiFePO4/FePO4 electrode. Desalination 546, 116188 (2023).
Zhang, Z. et al. Cross-linked PVDF-b-PAA composite binder enhanced LiMn2O4/C film based electrode for selective extraction of lithium from brine with a high Mg/Li ratio. Sep. Purif. Technol. 316, 123777 (2023).
Hu, B. et al. Lithium ion sieve modified three-dimensional graphene electrode for selective extraction of lithium by capacitive deionization. J. Colloid Interface Sci. 612, 392–400 (2022).
Zhao, X. Y. et al. Lithium extraction from brine in an ionic selective desalination battery. Desalination 481, 114360 (2020).
Zhao, X. Y., Yang, H. C., Wang, Y. F., Yang, L. B. & Zhu, L. Lithium extraction from brine by an asymmetric hybrid capacitor composed of heterostructured lithium-rich cathode and nano-bismuth anode. Sep. Purif. Technol. 274, 119078 (2021).
Zhao, A. L., Liu, J. C., Ai, X. P., Yang, H. X. & Cao, Y. L. Highly selective and pollution-free electrochemical extraction of lithium by a polyaniline/LixMn2O4 cell. ChemSusChem 12, 1361–1367 (2019).
Hoshino, T. Development of technology for recovering lithium from seawater by electrodialysis using ionic liquid membrane. Fusion Eng. Des. 88, 2956–2959 (2013).
Shen, K. X. et al. Flexible LATP composite membrane for lithium extraction from seawater via an electrochemical route. J. Membr. Sci. 671, 121358 (2023). This work used polymer poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) as the flexible framework and Li1.3Al0.3Ti1.7(PO4)3 (LATP) as the Li+ conductor to prepare a flexible composite membrane to extract lithium from seawater.
Abe, M. & Chitrakar, R. Synthetic inorganic ion-exchange materials. XLV. Recovery of lithium from seawater and hydrothermal water by titanium (IV) antimonate cation exchanger. Hydrometallurgy 19, 117–128 (1987).
Qiu, Z. W. et al. Li4Mn5O12 doped cellulose acetate membrane with low Mn loss and high stability for enhancing lithium extraction from seawater. Desalination 506, 115003 (2021).
Bao, L. R. et al. Preparation of Mg-doped Li1.6Mn1.6O4 with enhanced Li+ adsorption performance and anti-dissolution properties of Mn. Hydrometallurgy 209, 105772 (2022).
Qian, F. R. et al. Enhancing the Li+ adsorption and anti-dissolution properties of Li1.6Mn1.6O4 with Fe, Co doped. Hydrometallurgy 193, 105291 (2020).
Qian, F. R. et al. K-gradient doping to stabilize the spinel structure of Li1.6Mn1.6O4 for Li+ recovery. Dalton Trans. 49, 10939–10948 (2020).
Gao, Y. W. et al. Al and Cr ions co-doped spinel manganese lithium ion-sieve with enhanced Li+ adsorption performance and structural stability. Microporous Mesoporous Mater. 351, 112492 (2023).
Zhang, C. Y. et al. Lithium extraction from geothermal brine by granulated HTO titanium-based adsorbent with block-co-polymer poly (ethylene-co-vinyl alcohol) (EVAL) as binder. Chem. Eng. J. 467, 143526 (2023).
Zhong, J., Lin, S. & Yu, J. G. Effects of excessive lithium deintercalation on Li+ adsorption performance and structural stability of lithium/aluminum layered double hydroxides. J. Colloid Interface Sci. 572, 107–113 (2020).
Li, Y., Zhao, Y. J., Wang, H. Y. & Wang, M. The application of nanofiltration membrane for recovering lithium from salt lake brine. Desalination 468, 114081 (2019).
Wu, H. H. et al. Positively-charged PEI/TMC nanofiltration membrane prepared by adding a diamino-silane coupling agent for Li+/Mg2+ separation. J. Membr. Sci. 672, 121468 (2023).
Li, Q. et al. High performance Li+/Mg2+ separation membrane by grafted short chain amino-rich monomers. J. Membr. Sci. 677, 121634 (2023).
Zhang, S. Y. et al. Guanidyl-incorporated nanofiltration membranes toward superior Li+/Mg2+ selectivity under weakly alkaline environment. J. Membr. Sci. 663, 121063 (2022).
Peng, H. W., Liu, X. F., Su, Y. F., Li, J. P. & Zhao, Q. Advanced lithium extraction membranes derived from tagged-modification of polyamide networks. Angew. Chem. Int. Ed. 62, e202312795 (2023).
Hou, L. X. et al. Understanding the ion transport behavior across nanofluidic membranes in response to the charge variations. Adv. Funct. Mater. 31, 2009970 (2021).
Li, Q. et al. Ultrahigh-efficient separation of Mg2+/Li+ using an in-situ reconstructed positively charged nanofiltration membrane under an electric field. J. Membr. Sci. 641, 119880 (2022).
Luo, G. L. et al. Electrochemical lithium ions pump for lithium recovery from brine by using a surface stability Al2O3-ZrO2 coated LiMn2O4 electrode. J. Energy Chem. 69, 244–252 (2022).
Luo, G. L. et al. Electrochemical recovery lithium from brine via taming surface wettability of regeneration spent batteries cathode materials. Appl. Energy 337, 120890 (2023).
Du, X. et al. A novel electroactive λ-MnO2/PPy/PSS core–shell nanorod coated electrode for selective recovery of lithium ions at low concentration. J. Mater. Chem. A 4, 13989–13996 (2016).
Zhao, X. Y. et al. Efficient lithium extraction from brine using a three-dimensional nanostructured hybrid inorganic-gel framework electrode. ACS Sustain. Chem. Eng. 8, 4827–4837 (2020).
Zhao, X. Y., Gong, Y. X., Gao, K., Wang, Y. F. & Yang, H. Y. Tailored LMO@COF composite electrodes for direct electrochemical lithium extraction from high-temperature brines. Chem. Eng. J. 474, 145975 (2023).
Guo, Z. Y. et al. Effect of impurity ions in the electrosorption lithium extraction process: generation and restriction of “selective concentration polarization”. ACS Sustain. Chem. Eng. 8, 11834–11844 (2020).
Liu, X. H., Chen, X. Y., Zhao, Z. W. & Liang, X. X. Effect of Na+ on Li extraction from brine using LiFePO4/FePO4 electrodes. Hydrometallurgy 146, 24–28 (2014).
Trocoli, R., Battistel, A. & Mantia, F. L. Selectivity of a lithium-recovery process based on LiFePO4. Chem. Eur. J. 20, 9888–9891 (2014).
Lawagon, C. P. et al. Li1−xNi0.33Co1/3Mn1/3O2/Ag for electrochemical lithium recovery from brine. Chem. Eng. J. 348, 1000–1011 (2018).
Shang, X. H. et al. LiNi0.5Mn1.5O4-based hybrid capacitive deionization for highly selective adsorption of lithium from brine. Sep. Purif. Technol. 258, 118009 (2021).
Kim, N., Su, X. & Kim, C. Electrochemical lithium recovery system through the simultaneous lithium enrichment via sustainable redox reaction. Chem. Eng. J. 420, 127715 (2021).
Han, J. H. et al. Lithium and potassium resources of oilfield brine and development prospects in China. J. Salt Lake Res. 32, 90–100 (2024).
Grosjean, C., Miranda, P. H., Perrin, M. & Poggi, P. Assessment of world lithium resources and consequences of their geographic distribution on the expected development of the electric vehicle industry. Renew. Sustain. Energy Rev. 16, 1735–1744 (2012).
Marza, M. et al. Geological controls on lithium production from basinal brines across North America. J. Geochem. Explor. 257, 107383 (2024).
Engle, M. A. et al. Geochemistry of formation waters from the Wolfcamp and “Cline” shales: insights into brine origin, reservoir connectivity, and fluid flow in the Permian Basin, USA. Chem. Geol. 425, 76–92 (2016).
Collins, A. G. Geochemistry of liquids, gases, and rocks from the Smackover Formation. United States Department of the Interior (1974).
Edmunds, W. M., Kay, R. L. F. & McCartney, R. A. Origin of saline groundwaters in the Carnmenellis granite (Cornwall, England): natural processes and reaction during Hot Dry Rock reservoir circulation. Chem. Geol. 49, 287–301 (1985).
Hitchon, B., Underschultz, J. R. & Bachu, S. Industrial Mineral Potential of Alberta Formation Waters (Alberta Research Council, 1993).
Sanjuan, B. et al. Major geochemical characteristics of geothermal brines from the Upper Rhine Graben granitic basement with constraints on temperature and circulation. Chem. Geol. 428, 27–47 (2016).
Stober, I. Hydrochemical properties of deep carbonate aquifers in the SW German Molasse basin. Geotherm. Energy 2, 13 (2014).
Sanjuan, B. et al. Main geochemical characteristics of the deep geothermal brine at Vendenheim (Alsace, France) with constraints on temperature and fluid circulation. World Geothermal Congress (2021).
Setiawan, F. A., Rahayuningsih, E., Petrus, H. T. B. M., Nurpratama, M. I. & Perdana, I. Kinetics of silica precipitation in geothermal brine with seeds addition: minimizing silica scaling in a cold re-injection system. Geotherm. Energy 7, 22 (2019).
Somrani, A., Hamzaoui, A. H. & Pontie, M. Study on lithium separation from salt lake brines by nanofiltration (NF) and low pressure reverse osmosis (LPRO). Desalination 317, 184–192 (2013).
Regenspurg, S. et al. Geochemical properties of saline geothermal fluids from the in-situ geothermal laboratory Groß Schönebeck (Germany). Geochemistry 70, 3–12 (2010).
Zhang, R. et al. Extraction of boron from salt lake brine using 2-ethylhexanol. Hydrometallurgy 160, 129–136 (2016).
Yu, X. C., Wang, C. L., Huang, H., Wang, J. Y. & Yan, K. Lithium and brine geochemistry in the Qianjiang Formation of the Jianghan Basin, central China. Sci. Rep. 13, 4445 (2023).
Acknowledgements
This research was supported by the National Key R&D Program of China (2022YFB2502104), the National Natural Science Foundation of China (22239002, 22179059, 92372201), Key R&D Project financed by the Department of Science and Technology of Jiangsu Province (BE2020003) and the Science and Technology Innovation Fund for Emission Peak and Carbon Neutrality of Jiangsu Province (BK20231512, BK20220034).
Author information
Authors and Affiliations
Contributions
P.H. and S.Y. developed the framework of the manuscript. Y.W., H.P. and S.Y. collected the data. S.Y., P.H., H.P. and Y.W. wrote the manuscript. The project was supervised by P.H. and H.Z.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Daniel Alessi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Tables 1–8
This file provides a comprehensive dataset for the figures. These data are related to the operational conditions and efficacy of various lithium extraction methods, as well as the natural states and developmental status of the main lithium-containing water bodies worldwide. Supplementary Table 1: lithium resources. Information on lithium concentrations and Mg/Li ratios in various water bodies globally. These are categorized by reservoir types, such as salars, salt lakes and sedimentary waters, listing details such as the reservoir, country, lithium concentration in grams per litre (g l−1) and Mg/Li ratio. Supplementary Table 2: lithium extraction performance. Compares the feed and recovery solutions used/produced in lithium extraction methods, such as precipitation, solvent extraction, membrane-based separation, sorption and electrochemical-based separation, focusing on Mg/Li ratios and lithium concentrations. Supplementary Table 3: sorption capacities. Lists the sorption capacities of various sorbents used in lithium extraction, categorized by types such as LMO, LTO and Li/Al-LDH, with specific adsorption capacities noted in milligrams per gram (mg g−1). Supplementary Table 4: elemental loss rates of sorbents. Provides information on the dissolution elements and their loss rates in LTO-type and LMO-type sorbents, highlighting the loss rates of elements such as Mn and Ti over initial and subsequent cycles. Supplementary Table 5: membrane selectivity. Details on the selectivity factor of different membranes and the corresponding working conditions (Mg/Li ratio of the feed solution). Supplementary Table 6: extraction capacities of electrode materials. Lithium extraction capacities of various electrode materials, crucial for evaluating the efficiency of different extraction technologies. Supplementary Table 7: development status of various lithium-containing water bodies. Summarizes the lithium reserves of the main lithium-containing water bodies, their current developmental status for lithium extraction and the technologies used. Supplementary Table 8: summary of the main lithium-containing water bodies. Details of the Li+ concentration, Mg/Li ratio and locations of the main lithium-containing water bodies.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, S., Wang, Y., Pan, H. et al. Lithium extraction from low-quality brines. Nature 636, 309–321 (2024). https://doi.org/10.1038/s41586-024-08117-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-024-08117-1
This article is cited by
-
Constructing lithium-exclusive pathways in LiFePO4 electrodes
Nature Water (2025)
-
Lithium extraction by extractant confined in Pickering emulsion
Nature Communications (2025)
-
Adsorption-responsive bionic photothermal ion pump for reversible seawater lithium extraction
Nature Communications (2025)
-
Synthesis of [100]-only LiFePO4 nanosheets for efficient electrochemical lithium extraction from low-grade brines
Nature Water (2025)
-
Randomly oriented covalent organic framework membrane for selective Li+ sieving from other ions
Nature Communications (2025)