Abstract
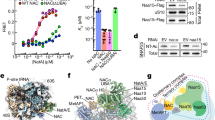
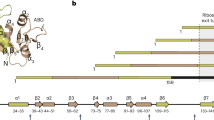
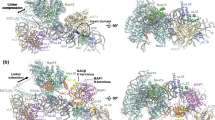
The nascent polypeptide-associated complex (NAC) is a conserved ribosome-bound factor with essential yet incompletely understood roles in protein biogenesis1. Here, we show that NAC is a multifaceted regulator that coordinates translation elongation, cotranslational folding, and organelle targeting through distinct interactions with nascent polypeptides both inside and outside the ribosome exit tunnel. Using NAC-selective ribosome profiling in C. elegans, we identify thousands of sequence-specific NAC binding events across the nascent proteome, revealing broad cotranslational engagement with hydrophobic and helical motifs in cytosolic, nuclear, ER, and mitochondrial proteins. Unexpectedly, we discover an intra-tunnel sensing mode, where NAC engages ribosomes with extremely short nascent polypeptides inside the exit tunnel in a sequence-specific manner. Moreover, initial NAC interactions induce an early elongation slowdown that tunes ribosome flux and prevent ribosome collisions, linking NAC’s chaperone activity to kinetic control of translation. We propose NAC action protects aggregation-prone intermediates by shielding amphipathic helices, thus promoting cytonuclear folding. NAC also supports mitochondrial membrane protein biogenesis and ER targeting by early recognition of signal sequences and transmembrane domain. Our findings establish NAC as an early-acting, multifaceted orchestrator of cotranslational proteostasis, with distinct mechanisms of action on nascent chains depending on their sequence features and subcellular destinations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Figures
Supplementary Figs 1–3.
Rights and permissions
About this article
Cite this article
Lee, J.H., Rabl, L., Gamerdinger, M. et al. NAC controls nascent chain fate through tunnel sensing and chaperone action. Nature (2025). https://doi.org/10.1038/s41586-025-10058-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41586-025-10058-2