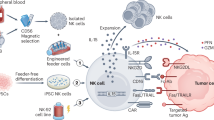
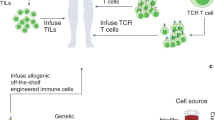
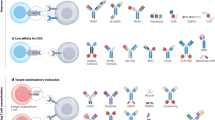
Abstract
Innate immune cells, including natural killer cells, macrophages and γδ T cells, are gaining prominence as promising candidates for cancer immunotherapy. Unlike conventional T cells, these cells possess attributes such as inherent antitumor activity, rapid immune responses, favorable safety profiles and the ability to target diverse malignancies without requiring prior antigen sensitization. In this Review, we examine the engineering strategies used to enhance their anticancer potential. We discuss challenges associated with each cell type and summarize insights from preclinical and clinical work. We propose strategies to address existing barriers, providing a perspective on the advancement of innate immune engineering as a powerful modality in anticancer treatment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Singh, A. K. & McGuirk, J. P. CAR T cells: continuation in a revolution of immunotherapy. Lancet Oncol. 21, e168–e178 (2020).
Pan, K. et al. CAR race to cancer immunotherapy: from CAR T, CAR NK to CAR macrophage therapy. J. Exp. Clin. Cancer Res. 41, 119 (2022).
Brudno, J. N. & Kochenderfer, J. N. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 127, 3321–3330 (2016).
Watanabe, N. & Mamonkin, M. Off-the-shelf chimeric antigen receptor T cells: how do we get there? Cancer J. 27, 176–181 (2021).
Vivier, E., Tomasello, E., Baratin, M., Walzer, T. & Ugolini, S. Functions of natural killer cells. Nat. Immunol. 9, 503–510 (2008).
Hadiloo, K., Tahmasebi, S. & Esmaeilzadeh, A. CAR-NKT cell therapy: a new promising paradigm of cancer immunotherapy. Cancer Cell Int. 23, 86 (2023).
Peng, L., Sferruzza, G., Yang, L., Zhou, L. & Chen, S. CAR-T and CAR-NK as cellular cancer immunotherapy for solid tumors. Cell Mol. Immunol. 10, 1089–1108 (2024).
Stögerer, T. & Stäger, S. Innate immune sensing by cells of the adaptive immune system. Front. Immunol. 11, 1081 (2020).
Courtney, A. N., Tian, G. & Metelitsa, L. S. Natural killer T cells and other innate-like T lymphocytes as emerging platforms for allogeneic cancer cell therapy. Blood 141, 869–876 (2023).
Jacquelot, N. et al. Innate lymphoid cells and cancer. Nat. Immunol. 23, 371–379 (2022).
Huntington, N. D., Vosshenrich, C. A. J. & Di Santo, J. P. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat. Rev. Immunol. 7, 703–714 (2007).
Rebuffet, L. et al. High-dimensional single-cell analysis of human natural killer cell heterogeneity. Nat. Immunol. 25, 1474–1488 (2024).
Fauriat, C., Long, E. O., Ljunggren, H. G. & Bryceson, Y. T. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood 115, 2167–2176 (2010).
Wagner, J. A. et al. CD56bright NK cells exhibit potent antitumor responses following IL-15 priming. J. Clin. Invest. 127, 4042–4058 (2017).
Bryceson, Y. T. et al. Molecular mechanisms of natural killer cell activation. J. Innate Immun. 3, 216–226 (2011).
Tassi, I., Klesney-Tait, J. & Colonna, M. Dissecting natural killer cell activation pathways through analysis of genetic mutations in human and mouse. Immunol. Rev. 214, 92–105 (2006).
Coënon, L. & Villalba, M. From CD16a biology to antibody-dependent cell-mediated cytotoxicity improvement. Front. Immunol. 13, 913215 (2022).
Sen Santara, S. et al. The NK cell receptor NKp46 recognizes ecto-calreticulin on ER-stressed cells. Nature 616, 348–356 (2023).
Campbell, K. S. & Purdy, A. K. Structure/function of human killer cell immunoglobulin-like receptors: lessons from polymorphisms, evolution, crystal structures and mutations. Immunology 132, 315–325 (2011).
Li, Y. et al. Unlocking the therapeutic potential of the NKG2A–HLA-E immune checkpoint pathway in T cells and NK cells for cancer immunotherapy. J. Immunother. Cancer 12, e009934 (2024).
O’Leary, J. G., Goodarzi, M., Drayton, D. L. & von Andrian, U. H. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat. Immunol. 7, 507–516 (2006).
Schlums, H. et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity 42, 443–456 (2015).
Cooper, M. A. et al. Cytokine-induced memory-like natural killer cells. Proc. Natl Acad. Sci. USA 106, 1915–1919 (2009).
Romee, R. et al. Cytokine activation induces human memory-like NK cells. Blood 120, 4751–4760 (2012).
Romee, R. et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. 8, 357ra123 (2016).
Shapiro, R. M. et al. Expansion, persistence, and efficacy of donor memory-like NK cells infused for posttransplant relapse. J. Clin. Invest. 132, e154334 (2022).
Berrien-Elliott, M. M. et al. Multidimensional analyses of donor memory-like NK cells reveal new associations with response after adoptive immunotherapy for leukemia. Cancer Discov. 10, 1854–1871 (2020).
Moscarelli, J., Zahavi, D., Maynard, R. & Weiner, L. M. The next generation of cellular immunotherapy: chimeric antigen receptor-natural killer cells. Transplant Cell Ther. 28, 650–656 (2022).
Tarannum, M., Romee, R. & Shapiro, R. M. Innovative strategies to improve the clinical application of NK cell-based immunotherapy. Front. Immunol. 13, 859177 (2022).
Li, T. et al. CAR-NK cells for cancer immunotherapy: recent advances and future directions. Front. Immunol. 15, 1361194 (2024).
Kong, J. C. et al. Chimeric antigen receptor-natural killer cell therapy: current advancements and strategies to overcome challenges. Front. Immunol. 15, 1384039 (2024).
Colamartino, A. B. L. et al. Efficient and robust NK-cell transduction with baboon envelope pseudotyped lentivector. Front. Immunol. 10, 2873 (2019).
Dong, H. et al. Memory-like NK cells armed with a neoepitope-specific CAR exhibit potent activity against NPM1 mutated acute myeloid leukemia. Proc. Natl Acad. Sci. USA 119, e2122379119 (2022).
Tarannum, M. et al. CAR memory-like NK cells targeting the membrane proximal domain of mesothelin demonstrate promising activity in ovarian cancer. Sci. Adv. 10, eadn0881 (2024).
Maia, A., Tarannum, M. & Romee, R. Genetic manipulation approaches to enhance the clinical application of NK cell-based immunotherapy. Stem Cells Transl. Med. 13, 230–242 (2024).
Zhang, B. et al. Chimeric antigen receptor-based natural killer cell immunotherapy in cancer: from bench to bedside. Cell Death Dis. 15, 50 (2024).
Acharya, S. et al. CD28 costimulation augments CAR signaling in NK cells via the LCK/CD3ζ/ZAP70 signaling axis. Cancer Discov. 14, 1879–1900 (2024).
Li, Y., Hermanson, D. L., Moriarity, B. S. & Kaufman, D. S. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell 23, 181–192 (2018).
Wang, W. et al. Breakthrough of solid tumor treatment: CAR-NK immunotherapy. Cell Death Discov. 10, 40 (2024).
Liu, E. et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 32, 520–531 (2018).
Chu, Y. et al. Anti-CD20 chimeric antigen receptor (CAR) modified expanded natural killer (NK) cells significantly mediate rituximab sensitive and resistant Burkitt lymphoma (BL) regression and improve survival in human BL xenografted NSG mice. Biol. Blood Marrow Transplant. 20, S257 (2014).
Caruso, S. et al. Safe and effective off-the-shelf immunotherapy based on CAR.CD123-NK cells for the treatment of acute myeloid leukaemia. J. Hematol. Oncol. 15, 163 (2022).
Troy, E. et al. CD38-CAR human NK cells in combination with ATRA enhance cytotoxicity against CD38-expressing hematologic malignancies. Blood Neoplasia 1, 100032 (2024).
Jacobs, M. T. et al. Memory-like differentiation, tumor-targeting mAbs, and chimeric antigen receptors enhance natural killer cell responses to head and neck cancer. Clin. Cancer Res. 29, 4196–4208 (2023).
Poorebrahim, M. et al. TCR-like CARs and TCR-CARs targeting neoepitopes: an emerging potential. Cancer Gene Ther. 28, 581–589 (2021).
Cichocki, F. et al. Dual antigen-targeted off-the-shelf NK cells show durable response and prevent antigen escape in lymphoma and leukemia. Blood 140, 2451–2462 (2022).
Rosenberg, S. A. & Dudley, M. E. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr. Opin. Immunol. 21, 233–240 (2009).
Wittibschlager, V. et al. CAR T-cell persistence correlates with improved outcome in patients with B-cell lymphoma. Int. J. Mol. Sci. 24, 5688 (2023).
Miller, J. S. et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 105, 3051–3057 (2005).
Mantesso, S., Geerts, D., Spanholtz, J. & Kučerová, L. Genetic engineering of natural killer cells for enhanced antitumor function. Front. Immunol. 11, 607131 (2020).
Carson, W. E. et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J. Exp. Med. 180, 1395–1403 (1994).
Ma, S., Caligiuri, M. A. & Yu, J. Harnessing IL-15 signaling to potentiate NK cell-mediated cancer immunotherapy. Trends Immunol. 43, 833–847 (2022).
Van den Eynde, A. et al. IL-15-secreting CAR natural killer cells directed toward the pan-cancer target CD70 eliminate both cancer cells and cancer-associated fibroblasts. J. Hematol. Oncol. 17, 8 (2024).
Marin, D. et al. Safety, efficacy and determinants of response of allogeneic CD19-specific CAR-NK cells in CD19+ B cell tumors: a phase 1/2 trial. Nat. Med. 30, 772–784 (2024).
Shanley, M. et al. Interleukin-21 engineering enhances NK cell activity against glioblastoma via CEBPD. Cancer Cell 42, 1450–1466 (2024).
Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 11, 3801 (2020).
Cao, Y. et al. Immune checkpoint molecules in natural killer cells as potential targets for cancer immunotherapy. Signal Transduct. Target. Ther. 5, 250 (2020).
Borst, L., van der Burg, S. H. & van Hall, T. The NKG2A–HLA-E axis as a novel checkpoint in the tumor microenvironment. Clin. Cancer Res. 26, 5549–5556 (2020).
Bexte, T. et al. CRISPR/Cas9 editing of NKG2A improves the efficacy of primary CD33-directed chimeric antigen receptor natural killer cells. Nat. Commun. 15, 8439 (2024).
Bexte, T. et al. CRISPR–Cas9 based gene editing of the immune checkpoint NKG2A enhances NK cell mediated cytotoxicity against multiple myeloma. Oncoimmunology 11, 2081415 (2022).
Qin, Y. et al. Developing enhanced immunotherapy using NKG2A knockout human pluripotent stem cell-derived NK cells. Cell Rep. 43, 114867 (2024).
Gong, Y. et al. NKG2A genetic deletion promotes human primary NK cell anti-tumor responses better than an anti-NKG2A monoclonal antibody. Mol. Ther. 32, 2711–2727 (2024).
Kaulfuss, M. et al. The NK cell checkpoint NKG2A maintains expansion capacity of human NK cells. Sci. Rep. 13, 10555 (2023).
Slattery, K. et al. TGFβ drives NK cell metabolic dysfunction in human metastatic breast cancer. J. Immunother. Cancer 9, e002044 (2021).
Wong, J. K. M. et al. TGF-β signalling limits effector function capacity of NK cell anti-tumour immunity in human bladder cancer. EBioMedicine 104, 105176 (2024).
Shaim, H. et al. Targeting the αv integrin/TGF-β axis improves natural killer cell function against glioblastoma stem cells. J. Clin. Invest. 131, e142116 (2021).
Thangaraj, J. L., Coffey, M., Lopez, E. & Kaufman, D. S. Disruption of TGF-β signaling pathway is required to mediate effective killing of hepatocellular carcinoma by human iPSC-derived NK cells. Cell Stem Cell 31, 1327–1343 (2024).
Delconte, R. B. et al. CIS is a potent checkpoint in NK cell-mediated tumor immunity. Nat. Immunol. 17, 816–824 (2016).
Bernard, P.-L. et al. Targeting CISH enhances natural cytotoxicity receptor signaling and reduces NK cell exhaustion to improve solid tumor immunity. J. Immunother. Cancer 10, e004244 (2022).
Zhu, H. et al. Metabolic reprograming via deletion of CISH in human iPSC-derived NK cells promotes in vivo persistence and enhances anti-tumor activity. Cell Stem Cell 27, 224–237 (2020).
Chen, L. et al. CD38-mediated immunosuppression as a mechanism of tumor cell escape from PD-1/PD-L1 blockade. Cancer Discov. 8, 1156–1175 (2018).
Naik, J. et al. CD38 as a therapeutic target for adult acute myeloid leukemia and T-cell acute lymphoblastic leukemia. Haematologica 104, e100–e103 (2019).
Gurney, M. et al. CD38 knockout natural killer cells expressing an affinity optimized CD38 chimeric antigen receptor successfully target acute myeloid leukemia with reduced effector cell fratricide. Haematologica 107, 437–445 (2022).
Naeimi Kararoudi, M. et al. CD38 deletion of human primary NK cells eliminates daratumumab-induced fratricide and boosts their effector activity. Blood 136, 2416–2427 (2020).
Cichocki, F. et al. NK cells lacking CD38 are resistant to oxidative stress-induced death. Blood 134, 3215 (2019).
Peng, L. et al. In vivo AAV-SB-CRISPR screens of tumor-infiltrating primary NK cells identify genetic checkpoints of CAR-NK therapy. Nat. Biotechnol. https://doi.org/10.1038/s41587-024-02282-4 (2024).
Jo, D.-H. et al. Simultaneous engineering of natural killer cells for CAR transgenesis and CRISPR–Cas9 knockout using retroviral particles. Mol. Ther. Methods Clin. Dev. 29, 173–184 (2023).
Lupo, K. B. et al. synNotch-programmed iPSC-derived NK cells usurp TIGIT and CD73 activities for glioblastoma therapy. Nat. Commun. 15, 1909 (2024).
Chen, Q. et al. Fever-range thermal stress promotes lymphocyte trafficking across high endothelial venules via an interleukin 6 trans-signaling mechanism. Nat. Immunol. 7, 1299–1308 (2006).
Giraldo, N. A. et al. The clinical role of the TME in solid cancer. Br. J. Cancer 120, 45–53 (2019).
Yao, X. & Matosevic, S. Chemokine networks modulating natural killer cell trafficking to solid tumors. Cytokine Growth Factor Rev. 59, 36–45 (2021).
Ng, Y. Y., Du, Z., Zhang, X., Chng, W. J. & Wang, S. CXCR4 and anti-BCMA CAR co-modified natural killer cells suppress multiple myeloma progression in a xenograft mouse model. Cancer Gene Ther. 29, 475–483 (2022).
Sanz-Ortega, L., Andersson, A. & Carlsten, M. Harnessing upregulated E-selectin while enhancing SDF-1α sensing redirects infused NK cells to the AML-perturbed bone marrow. Leukemia 38, 579–589 (2024).
Samson, N. & Ablasser, A. The cGAS–STING pathway and cancer. Nat. Cancer 3, 1452–1463 (2022).
Knelson, E. H. et al. Activation of tumor-cell STING primes NK-cell therapy. Cancer Immunol. Res. 10, 947–961 (2022).
Yang, S. et al. Non-pathogenic E. coli displaying decoy-resistant IL18 mutein boosts anti-tumor and CAR NK cell responses. Nat. Biotechnol. https://doi.org/10.1038/s41587-024-02418-6 (2024).
Ruggeri, L. et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 295, 2097–2100 (2002).
Leung, W. Infusions of allogeneic natural killer cells as cancer therapy. Clin. Cancer Res. 20, 3390–3400 (2014).
Cooley, S. et al. First-in-human trial of rhIL-15 and haploidentical natural killer cell therapy for advanced acute myeloid leukemia. Blood Adv. 3, 1970–1980 (2019).
Tarannum, M. & Romee, R. Cytokine-induced memory-like natural killer cells for cancer immunotherapy. Stem Cell Res. Ther. 12, 592 (2021).
Keppel, M. P., Yang, L. & Cooper, M. A. Murine NK cell intrinsic cytokine-induced memory-like responses are maintained following homeostatic proliferation. J. Immunol. 190, 4754–4762 (2013).
Shapiro, R. M. et al. First-in-human evaluation of memory-like NK cells with an IL-15 super-agonist and CTLA-4 blockade in advanced head and neck cancer. J. Hematol. Oncol. 18, 17 (2025).
Denman, C. J. et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS ONE 7, e30264 (2012).
Liu, E. et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 382, 545–553 (2020).
Dhakal, B. et al. Interim phase I clinical data of FT576 as monotherapy and in combination with daratumumab in subjects with relapsed/refractory multiple myeloma. Blood 140, 4586–4587 (2022).
Cichocki, F. et al. Quadruple gene-engineered natural killer cells enable multi-antigen targeting for durable antitumor activity against multiple myeloma. Nat. Commun. 13, 7341 (2022).
Cichocki, F., van der Stegen, S. J. C. & Miller, J. S. Engineered and banked iPSCs for advanced NK- and T-cell immunotherapies. Blood 141, 846–855 (2023).
Ghobadi, A. et al. Induced pluripotent stem-cell-derived CD19-directed chimeric antigen receptor natural killer cells in B-cell lymphoma: a phase 1, first-in-human trial. Lancet 405, 127–136 (2025).
Li, Y. et al. KIR-based inhibitory CARs overcome CAR-NK cell trogocytosis-mediated fratricide and tumor escape. Nat. Med. 28, 2133–2144 (2022).
Hinte, L. C. et al. Adipose tissue retains an epigenetic memory of obesity after weight loss. Nature 636, 457–465 (2024).
Rosenberg, S. A., Restifo, N. P., Yang, J. C., Morgan, R. A. & Dudley, M. E. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat. Rev. Cancer 8, 299–308 (2008).
Zhang, W. & Huang, X. In vivo gene editing and in situ generation of chimeric antigen receptor cells for next-generation cancer immunotherapy. J. Hematol. Oncol. 17, 110 (2024).
Soroudi, S., Jaafari, M. R. & Arabi, L. Lipid nanoparticle (LNP) mediated mRNA delivery in cardiovascular diseases: advances in genome editing and CAR T cell therapy. J. Control. Release 372, 113–140 (2024).
Beltran-Garcia, J. et al. Development of novel lipid nanoparticles and virus-like particles for in vivo engineering of immune cells for targeted cancer therapy. Blood 142, 3632 (2023).
Barry, S. T., Gabrilovich, D. I., Sansom, O. J., Campbell, A. D. & Morton, J. P. Therapeutic targeting of tumour myeloid cells. Nat. Rev. Cancer 23, 216–237 (2023).
Vitale, I., Manic, G., Coussens, L. M., Kroemer, G. & Galluzzi, L. Macrophages and metabolism in the tumor microenvironment. Cell Metab. 30, 36–50 (2019).
Christofides, A. et al. The complex role of tumor-infiltrating macrophages. Nat. Immunol. 23, 1148–1156 (2022).
Casanova-Acebes, M. et al. Tissue-resident macrophages provide a pro-tumorigenic niche to early NSCLC cells. Nature 595, 578–584 (2021).
Qian, B. Z. et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475, 222–225 (2011).
Mantovani, A., Allavena, P., Marchesi, F. & Garlanda, C. Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov. 21, 799–820 (2022).
Wang, J., Zhu, N., Su, X., Gao, Y. & Yang, R. Novel tumor-associated macrophage populations and subpopulations by single cell RNA sequencing. Front. Immunol. 14, 1264774 (2023).
Chen, M. Y., Zhang, F., Goedegebuure, S. P. & Gillanders, W. E. Dendritic cell subsets and implications for cancer immunotherapy. Front. Immunol. 15, 1393451 (2024).
Cheng, S. et al. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell 184, 792–809 (2021).
Fridlender, Z. G. et al. Polarization of tumor-associated neutrophil phenotype by TGF-β: ‘N1’ versus ‘N2’ TAN. Cancer Cell 16, 183–194 (2009).
van Vlerken-Ysla, L., Tyurina, Y. Y., Kagan, V. E. & Gabrilovich, D. I. Functional states of myeloid cells in cancer. Cancer Cell 41, 490–504 (2023).
Tugues, S. et al. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 22, 237–246 (2015).
Kaczanowska, S. et al. Genetically engineered myeloid cells rebalance the core immune suppression program in metastasis. Cell 184, 2033–2052 (2021).
Ghasemi, A. et al. Cytokine-armed dendritic cell progenitors for antigen-agnostic cancer immunotherapy. Nat. Cancer 5, 240–261 (2024).
Kang, B. et al. Large-scale generation of IL-12 secreting macrophages from human pluripotent stem cells for cancer therapy. Mol. Ther. Methods Clin. Dev. 32, 101204 (2024).
Zitvogel, L., Galluzzi, L., Kepp, O., Smyth, M. J. & Kroemer, G. Type I interferons in anticancer immunity. Nat. Rev. Immunol. 15, 405–414 (2015).
De Palma, M. et al. Tumor-targeted interferon-α delivery by Tie2-expressing monocytes inhibits tumor growth and metastasis. Cancer Cell 14, 299–311 (2008).
Miyashita, A. et al. Immunotherapy against metastatic melanoma with human iPS cell-derived myeloid cell lines producing type I interferons. Cancer Immunol. Res. 4, 248–258 (2016).
Sloas, C., Gill, S. & Klichinsky, M. Engineered CAR-macrophages as adoptive immunotherapies for solid tumors. Front. Immunol. 12, 783305 (2021).
June, C. H. & Sadelain, M. Chimeric antigen receptor therapy. N. Engl. J. Med. 379, 64–73 (2018).
Zhang, L. et al. Pluripotent stem cell-derived CAR-macrophage cells with antigen-dependent anti-cancer cell functions. J. Hematol. Oncol. 13, 153 (2020).
Morrissey, M. A. et al. Chimeric antigen receptors that trigger phagocytosis. eLife 7, e36688 (2018).
Biglari, A., Southgate, T. D., Fairbairn, L. J. & Gilham, D. E. Human monocytes expressing a CEA-specific chimeric CD64 receptor specifically target CEA-expressing tumour cells in vitro and in vivo. Gene Ther. 13, 602–610 (2006).
Niu, Z. et al. Chimeric antigen receptor-modified macrophages trigger systemic anti-tumour immunity. J. Pathol. 253, 247–257 (2021).
Klichinsky, M. et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 38, 947–953 (2020).
Zhang, W. et al. Chimeric antigen receptor macrophage therapy for breast tumours mediated by targeting the tumour extracellular matrix. Br. J. Cancer 121, 837–845 (2019).
Chen, Y. et al. The application of HER2 and CD47 CAR-macrophage in ovarian cancer. J. Transl. Med. 21, 654 (2023).
Shen, J. et al. Activating innate immune responses repolarizes hPSC-derived CAR macrophages to improve anti-tumor activity. Cell Stem Cell 31, 1003–1019 (2024).
Lei, A. et al. A second-generation M1-polarized CAR macrophage with antitumor efficacy. Nat. Immunol. 25, 102–116 (2024).
Yang, Z. et al. Dual mRNA co-delivery for in situ generation of phagocytosis-enhanced CAR macrophages augments hepatocellular carcinoma immunotherapy. J. Control. Release 360, 718–733 (2023).
Chen, C. et al. Intracavity generation of glioma stem cell-specific CAR macrophages primes locoregional immunity for postoperative glioblastoma therapy. Sci. Transl. Med. 14, eabn1128 (2022).
Wang, X. et al. Metabolic reprogramming via ACOD1 depletion enhances function of human induced pluripotent stem cell-derived CAR-macrophages in solid tumors. Nat. Commun. 14, 5778 (2023).
Wu, J. et al. Targeted glycan degradation potentiates cellular immunotherapy for solid tumors. Proc. Natl Acad. Sci. USA 120, e2300366120 (2023).
Shah, Z. et al. Human anti-PSCA CAR macrophages possess potent antitumor activity against pancreatic cancer. Cell Stem Cell 31, 803–817 (2024).
Rodewald, H. R., Arulanandam, A. R., Koyasu, S. & Reinherz, E. L. The high affinity Fcε receptor γ subunit (Fcε RI γ) facilitates T cell receptor expression and antigen/major histocompatibility complex-driven signaling in the absence of CD3ζ and CD3η. J. Biol. Chem. 266, 15974–15978 (1991).
Yudushkin, I. A. & Vale, R. D. Imaging T-cell receptor activation reveals accumulation of tyrosine-phosphorylated CD3ζ in the endosomal compartment. Proc. Natl Acad. Sci. USA 107, 22128–22133 (2010).
Haruta, M. et al. TAP-deficient human iPS cell-derived myeloid cell lines as unlimited cell source for dendritic cell-like antigen-presenting cells. Gene Ther. 20, 504–513 (2013).
Chang, Y. et al. Engineering chimeric antigen receptor neutrophils from human pluripotent stem cells for targeted cancer immunotherapy. Cell Rep. 40, 111128 (2022).
Kon, E., Ad-El, N., Hazan-Halevy, I., Stotsky-Oterin, L. & Peer, D. Targeting cancer with mRNA-lipid nanoparticles: key considerations and future prospects. Nat. Rev. Clin. Oncol. 20, 739–754 (2023).
Kirschenbaum, D. et al. Time-resolved single-cell transcriptomics defines immune trajectories in glioblastoma. Cell 187, 149–165 (2024).
Kloosterman, D. J. & Akkari, L. Macrophages at the interface of the co-evolving cancer ecosystem. Cell 186, 1627–1651 (2023).
O’Neill, L. A. J. & Artyomov, M. N. Itaconate: the poster child of metabolic reprogramming in macrophage function. Nat. Rev. Immunol. 19, 273–281 (2019).
Chen, Y. J. et al. Targeting IRG1 reverses the immunosuppressive function of tumor-associated macrophages and enhances cancer immunotherapy. Sci. Adv. 9, eadg0654 (2023).
Logtenberg, M. E. W., Scheeren, F. A. & Schumacher, T. N. The CD47–SIRPα immune checkpoint. Immunity 52, 742–752 (2020).
Wang, X., Zhang, S., Xue, D., Neculai, D. & Zhang, J. Metabolic reprogramming of macrophages in cancer therapy. Trends Endocrinol. Metab. https://doi.org/10.1016/j.tem.2024.08.009 (2024).
Zhang, H. et al. Silencing of SIRPα enhances the antitumor efficacy of CAR-M in solid tumors. Cell Mol. Immunol. 21, 1335–1349 (2024).
Heras-Murillo, I., Adan-Barrientos, I., Galan, M., Wculek, S. K. & Sancho, D. Dendritic cells as orchestrators of anticancer immunity and immunotherapy. Nat. Rev. Clin. Oncol. 21, 257–277 (2024).
Handy, C. E. & Antonarakis, E. S. Sipuleucel-T for the treatment of prostate cancer: novel insights and future directions. Future Oncol. 14, 907–917 (2018).
Andreesen, R., Hennemann, B. & Krause, S. W. Adoptive immunotherapy of cancer using monocyte-derived macrophages: rationale, current status, and perspectives. J. Leukoc. Biol. 64, 419–426 (1998).
Chernykh, E. R. et al. Safety and therapeutic potential of M2 macrophages in stroke treatment. Cell Transplant. 25, 1461–1471 (2016).
Moroni, F. et al. Safety profile of autologous macrophage therapy for liver cirrhosis. Nat. Med. 25, 1560–1565 (2019).
Reiss, K. A. et al. A phase 1, first-in-human (FIH) study of the anti-HER2 CAR macrophage CT-0508 in subjects with HER2 overexpressing solid tumors. J. Clin. Oncol. 40, 2533 (2022).
Reiss, K. A. et al. CAR-macrophage therapy for HER2-overexpressing advanced solid tumors: a phase 1 trial. Nat. Med. https://doi.org/10.1038/s41591-025-03495-z (2025).
Li, X. et al. A clinical study of autologous chimeric antigen receptor macrophage targeting mesothelin shows safety in ovarian cancer therapy. J. Hematol. Oncol. 17, 116 (2024).
Pierini, S. et al. Chimeric antigen receptor macrophages (CAR-M) sensitize HER2+ solid tumors to PD1 blockade in pre-clinical models. Nat. Commun. 16, 706 (2025).
Abdou, Y. et al. A phase 1, first-in-human (FIH) study of autologous macrophages engineered to express an anti-HER2 chimeric antigen receptor (CAR) in participants (pts) with HER2-overexpressing solid tumors. J. Clin. Oncol. 41, TPS2666 (2023).
Lovgren, T. et al. Complete and long-lasting clinical responses in immune checkpoint inhibitor-resistant, metastasized melanoma treated with adoptive T cell transfer combined with DC vaccination. Oncoimmunology 9, 1792058 (2020).
Guo, Z. et al. Durable complete response to neoantigen-loaded dendritic-cell vaccine following anti-PD-1 therapy in metastatic gastric cancer. NPJ Precis Oncol. 6, 34 (2022).
van Willigen, W. W. et al. Response and survival of metastatic melanoma patients treated with immune checkpoint inhibition for recurrent disease on adjuvant dendritic cell vaccination. Oncoimmunology 9, 1738814 (2020).
Rob, L. et al. Safety and efficacy of dendritic cell-based immunotherapy DCVAC/OvCa added to first-line chemotherapy (carboplatin plus paclitaxel) for epithelial ovarian cancer: a phase 2, open-label, multicenter, randomized trial. J. Immunother. Cancer 10, e003190 (2022).
Rodriguez-Ruiz, M. E. et al. Combined immunotherapy encompassing intratumoral poly-ICLC, dendritic-cell vaccination and radiotherapy in advanced cancer patients. Ann. Oncol. 29, 1312–1319 (2018).
Blumenthal, D. et al. Pre-clinical development of CAR monocytes (CAR Mono) for solid tumor immunotherapy. Cancer Res. 82, 582 (2022).
Abdou, Y. et al. A phase 1, first-in-human study of autologous monocytes engineered to express an anti-HER2 chimeric antigen receptor (CAR) in participants with HER2-overexpressing solid tumors. J. Clin. Oncol. 42, TPS2682 (2024).
Xin, W. et al. Structures of human γδ T cell receptor–CD3 complex. Nature 630, 222–229 (2024).
Hayday, A., Dechanet-Merville, J., Rossjohn, J. & Silva-Santos, B. Cancer immunotherapy by γδ T cells. Science 386, eabq7248 (2024).
Hu, Y. et al. T cells: origin and fate, subsets, diseases and immunotherapy. Signal Transduct. Target. Ther. 8, 434 (2023).
Fournié, J. J. & Bonneville, M. Stimulation of γδ T cells by phosphoantigens. Res. Immunol. 147, 338–347 (1996).
Karunakaran, M. M. et al. Butyrophilin-2A1 directly binds germline-encoded regions of the Vγ9Vδ2 TCR and is essential for phosphoantigen sensing. Immunity 52, 487–498 (2020).
Willcox, C. R. et al. Butyrophilin-like 3 directly binds a human Vγ4+ T cell receptor using a modality distinct from clonally-restricted antigen. Immunity 51, 813–825 (2019).
Willcox, C. R. et al. Phosphoantigen sensing combines TCR-dependent recognition of the BTN3A IgV domain and germline interaction with BTN2A1. Cell Rep. 42, 112321 (2023).
Rigau, M. et al. Butyrophilin 2A1 is essential for phosphoantigen reactivity by γδ T cells. Science 367, eaay5516 (2020).
Fulford, T. S. et al. Vγ9Vδ2 T cells recognize butyrophilin 2A1 and 3A1 heteromers. Nat. Immunol. 25, 1355–1366 (2024).
Yuan, L. et al. Phosphoantigens glue butyrophilin 3A1 and 2A1 to activate Vγ9Vδ2 T cells. Nature 621, 840–848 (2023).
Uldrich, A. P. et al. CD1d-lipid antigen recognition by the γδ TCR. Nat. Immunol. 14, 1137–1145 (2013).
Roy, S. et al. Molecular analysis of lipid-reactive Vδ1 γδ T cells identified by CD1c tetramers. J. Immunol. 196, 1933–1942 (2016).
Reijneveld, J. F. et al. Human γδ T cells recognize CD1b by two distinct mechanisms. Proc. Natl Acad. Sci. USA 117, 22944–22952 (2020).
Wegrecki, M. et al. Atypical sideways recognition of CD1a by autoreactive γδ T cell receptors. Nat. Commun. 13, 3872 (2022).
Benveniste, P. M. et al. Generation and molecular recognition of melanoma-associated antigen-specific human γδ T cells. Sci. Immunol. 3, eaav4036 (2018).
Marlin, R. et al. Sensing of cell stress by human γδ TCR-dependent recognition of annexin A2. Proc. Natl Acad. Sci. USA 114, 3163–3168 (2017).
Willcox, C. R. et al. Cytomegalovirus and tumor stress surveillance by binding of a human γδ T cell antigen receptor to endothelial protein C receptor. Nat. Immunol. 13, 872–879 (2012).
Hayday, A. C. γδ T cells and the lymphoid stress-surveillance response. Immunity 31, 184–196 (2009).
Ravens, S. et al. Human γδ T cells are quickly reconstituted after stem-cell transplantation and show adaptive clonal expansion in response to viral infection. Nat. Immunol. 18, 393–401 (2017).
Davey, M. S. et al. Clonal selection in the human Vδ1 T cell repertoire indicates γδ TCR-dependent adaptive immune surveillance. Nat. Commun. 8, 14760 (2017).
Davey, M. S. et al. The human Vδ2+ T-cell compartment comprises distinct innate-like Vγ9+ and adaptive Vγ9– subsets. Nat. Commun. 9, 1760 (2018).
Vantourout, P. & Hayday, A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat. Rev. Immunol. 13, 88–100 (2013).
Hayday, A. C. & Vantourout, P. The innate biologies of adaptive antigen receptors. Annu. Rev. Immunol. 38, 487–510 (2020).
Fisher, J. P. H. et al. Neuroblastoma killing properties of Vδ2 and Vδ2-negative γδT cells following expansion by artificial antigen-presenting cells. Clin. Cancer Res. 20, 5720–5732 (2014).
Fowler, D. et al. Payload-delivering engineered γδ T cells display enhanced cytotoxicity, persistence, and efficacy in preclinical models of osteosarcoma. Sci. Transl. Med. 16, eadg9814 (2024).
Angelini, D. F. et al. FcγRIII discriminates between 2 subsets of Vγ9Vδ2 effector cells with different responses and activation pathways. Blood 104, 1801–1807 (2004).
Brandes, M., Willimann, K. & Moser, B. Professional antigen-presentation function by human γδ T cells. Science 309, 264–268 (2005).
Himoudi, N. et al. Human γδ T lymphocytes are licensed for professional antigen presentation by interaction with opsonized target cells. J. Immunol. 188, 1708–1716 (2012).
Hurtado, M. O. et al. Tumor infiltrating lymphocytes expanded from pediatric neuroblastoma display heterogeneity of phenotype and function. PLoS ONE 14, e0216373 (2019).
Gentles, A. J. et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 21, 938–945 (2015).
Thorsson, V. et al. The immune landscape of cancer. Immunity 48, 812–830 (2018).
Rancan, C. et al. Exhausted intratumoral Vδ2− γδ T cells in human kidney cancer retain effector function. Nat. Immunol. 24, 612–624 (2023).
Wu, Y. et al. A local human Vδ1 T cell population is associated with survival in nonsmall-cell lung cancer. Nat. Cancer 3, 696–709 (2022).
de Vries, N. L. et al. γδ T cells are effectors of immunotherapy in cancers with HLA class I defects. Nature 613, 743–750 (2023).
Almeida, A. R. et al. Delta one T cells for immunotherapy of chronic lymphocytic leukemia: clinical-grade expansion/differentiation and preclinical proof of concept. Clin. Cancer Res. 22, 5795–5804 (2016).
Ferry, G. M. et al. A simple and robust single-step method for CAR-Vδ1 γδT cell expansion and transduction for cancer immunotherapy. Front. Immunol. 13, 863155 (2022).
López-Cantillo, G., Urueña, C., Camacho, B. A. & Ramírez-Segura, C. CAR-T cell performance: how to improve their persistence? Front. Immunol. 13, 878209 (2022).
Saura-Esteller, J. et al. γδT-cell based cancer immunotherapy: past-present-future. Front. Immunol. 13, 915837 (2022).
Anderson, N. D. et al. Transcriptional signatures associated with persisting CD19 CAR-T cells in children with leukemia. Nat. Med. 29, 1700–1709 (2023).
Melenhorst, J. J. et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature 602, 503–509 (2022).
Deniger, D. C. et al. Activating and propagating polyclonal γδ T cells with broad specificity for malignancies. Clin. Cancer Res. 20, 5708–5719 (2014).
Deniger, D. C. et al. Bispecific T-cells expressing polyclonal repertoire of endogenous γδ T-cell receptors and introduced CD19-specific chimeric antigen receptor. Mol. Ther. 21, 638–647 (2013).
Fisher, J. et al. Avoidance of on-target off-tumor activation using a co-stimulation-only chimeric antigen receptor. Mol. Ther. 25, 1234–1247 (2017).
Fisher, J. et al. Engineering γδT cells limits tonic signaling associated with chimeric antigen receptors. Sci. Signal 12, eaax1872 (2019).
Fleischer, L. C. et al. Non-signaling chimeric antigen receptors enhance antigen-directed killing by γδ T cells in contrast to αβ T cells. Mol. Ther. Oncolytics 18, 149–160 (2020).
Harrer, D. C. et al. RNA-transfection of γ/δ T cells with a chimeric antigen receptor or an α/β T-cell receptor: a safer alternative to genetically engineered α/β T cells for the immunotherapy of melanoma. BMC Cancer 17, 551 (2017).
Ang, W. X. et al. Electroporation of NKG2D RNA CAR improves Vγ9Vδ2 T cell responses against human solid tumor xenografts. Mol. Ther. Oncolytics 17, 421–430 (2020).
Capsomidis, A. et al. Chimeric antigen receptor-engineered human γδ T cells: enhanced cytotoxicity with retention of cross presentation. Mol. Ther. 26, 354–365 (2018).
Zhang, X. et al. A CD123-specific chimeric antigen receptor augments anti-acute myeloid leukemia activity of Vγ9Vδ2 T cells. Immunotherapy 14, 321–336 (2022).
Zhai, X. et al. MUC1-Tn-targeting chimeric antigen receptor-modified Vγ9Vδ2 T cells with enhanced antigen-specific anti-tumor activity. Am. J. Cancer Res. 11, 79–91 (2020).
Zhang, X. et al. Vγ9Vδ2 T cells expressing a BCMA-specific chimeric antigen receptor inhibit multiple myeloma xenograft growth. PLoS ONE 17, e0267475 (2022).
Wang, Y. et al. B7H3-targeting chimeric antigen receptor modification enhances antitumor effect of Vγ9Vδ2 T cells in glioblastoma. J. Transl. Med. 21, 672 (2023).
Makkouk, A. et al. 119 ADI-002: an IL-15 armored allogeneic ‘off-the-shelf’ Vδ1 γδ CAR T cell therapy for solid tumors targeting glypican-3 (GPC3). J. Immunother. Cancer 9, A128 (2021).
Dong, R., Zhang, Y., Huang, H., Zeng, X. & Xiao, H. A novel strategy to produce CAR-γδ T cells via targeted knockout by CRISPR/Cas9 and targeted knockin by AAV. Blood 142, 6840 (2023).
Themeli, M. et al. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat. Biotechnol. 31, 928–933 (2013).
Wallet, M. A. et al. Induced pluripotent stem cell-derived γδ CAR-T cells for cancer immunotherapy. Blood 138, 2771 (2021).
Neelapu, S. S. et al. A phase 1 safety and efficacy study of ADI-001 anti-CD20 CAR-engineered allogeneic γδ T cells in adults with B cell malignancies, in monotherapy and combination with IL-2. Blood 138, 2834 (2021).
Nishimoto, K. P. et al. Allogeneic CD20‐targeted γδ T cells exhibit innate and adaptive antitumor activities in preclinical B‐cell lymphoma models. Clin. Transl. Immunol. 11, e1373 (2022).
Frieling, J. S. et al. γδ-Enriched CAR-T cell therapy for bone metastatic castrate-resistant prostate cancer. Sci. Adv. 9, eadf0108 (2023).
Themeli, M. & Sadelain, M. Combinatorial antigen targeting: ideal T-cell sensing and anti-tumor response. Trends Mol. Med. 22, 271–273 (2016).
Acker, H. H. V. et al. Interleukin-15 enhances the proliferation, stimulatory phenotype, and antitumor effector functions of human γδ T cells. J. Hematol. Oncol. 9, 101 (2016).
Makkouk, A. et al. Off-the-shelf Vδ1 γδ T cells engineered with glypican-3 (GPC-3)-specific chimeric antigen receptor (CAR) and soluble IL-15 display robust antitumor efficacy against hepatocellular carcinoma. J. Immunother. Cancer 9, e003441 (2021).
Hurton, L. V. et al. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc. Natl Acad. Sci. USA 113, E7788–E7797 (2016).
Morgan, R. A. et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 314, 126–129 (2006).
Kawai, A. et al. Safety and efficacy of NY-ESO-1 antigen-specific T-cell receptor gene-transduced T lymphocytes in patients with synovial sarcoma: a phase I/II clinical trial. Clin. Cancer Res. 29, 5069–5078 (2023).
Thomas, S. et al. Framework engineering to produce dominant T cell receptors with enhanced antigen-specific function. Nat. Commun. 10, 4451 (2019).
van der Veken, L. T. et al. αβ T-cell receptor engineered γδ T cells mediate effective antileukemic reactivity. Cancer Res. 66, 3331–3337 (2006).
Hiasa, A. et al. Rapid αβ TCR-mediated responses in γδ T cells transduced with cancer-specific TCR genes. Gene Ther. 16, 620–628 (2009).
Marcu-Malina, V. et al. Redirecting αβ T cells against cancer cells by transfer of a broadly tumor-reactive γδT-cell receptor. Blood 118, 50–59 (2011).
Asbury, S., Yoo, S. M. & Bramson, J. 101 Engineering γδ T cells with the T-cell antigen coupler receptor effectively induces antigen-specific tumor cytotoxicity in vitro and in vivo. J. Immunother. Cancer 8, A63.2–A64 (2020).
van Diest, E. et al. γδ TCR anti-CD3 bispecific molecules (GABs) as novel immunotherapeutic compounds. J. Immunother. Cancer 9, e003850 (2021).
Dekkers, J. F. et al. Uncovering the mode of action of engineered T cells in patient cancer organoids. Nat. Biotechnol. 41, 60–69 (2023).
van Diest, E. et al. The making of multivalent γδ TCR anti-CD3 bispecific T cell engagers. Front. Immunol. 13, 1052090 (2023).
Vydra, J. et al. A phase I trial of allogeneic γδ T lymphocytes from haploidentical donors in patients with refractory or relapsed acute myeloid leukemia. Clin. Lymphoma Myeloma Leuk. 23, e232–e239 (2023).
Dimitri, A., Herbst, F. & Fraietta, J. A. Engineering the next-generation of CAR T-cells with CRISPR–Cas9 gene editing. Mol. Cancer 21, 78 (2022).
Chiesa, R. et al. Base-edited CAR7 T cells for relapsed T-cell acute lymphoblastic leukemia. N. Engl. J. Med. 389, 899–910 (2023).
Mo, F. et al. Engineered off-the-shelf therapeutic T cells resist host immune rejection. Nat. Biotechnol. 39, 56–63 (2021).
Gonzalez, H., Hagerling, C. & Werb, Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 32, 1267–1284 (2018).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
R.R. is cofounder of InnDura Therapeutics, is a Scientific Advisory Board member of Glycostem Therapeutics and receives funding from Parker Institute for Cancer Immunotherapy and Miltenyi Biotech. J.Z. is a scientific founder of CellOrigin Biotech. J.A. holds founder stock in Autolus, Ltd., and is the inventor on patents related to CAR T cell function.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tarannum, M., Ding, X., Barisa, M. et al. Engineering innate immune cells for cancer immunotherapy. Nat Biotechnol 43, 516–533 (2025). https://doi.org/10.1038/s41587-025-02629-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41587-025-02629-5
This article is cited by
-
Synergistic innate-adaptive immunity by NKG2D-specific CAR-macrophages drives durable remission in hepatocellular carcinoma
Molecular Cancer (2025)
-
TIM-3 blockade reverses oncolytic vaccinia virus-induced DCs inactivation and T cells exhaustion to improve antitumor immunity and therapeutic efficacy
Journal of Experimental & Clinical Cancer Research (2025)
-
Self-iterative multiple-instance learning enables the prediction of CD4+ T cell immunogenic epitopes
Nature Machine Intelligence (2025)
-
Label-free estimation of regulatory T cell activation markers using Raman spectroscopy with machine learning
Scientific Reports (2025)