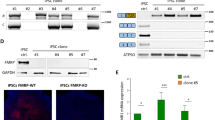
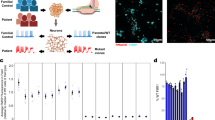
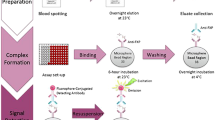
Abstract
Gene-silencing disorders, of which fragile X syndrome (FXS) is the most prevalent, are diseases caused by a blockade of gene transcription, usually due to DNA hypermethylation. FXS is a common form of inherited intellectual disability and autism. Unlike most hereditary diseases driven by mutations within the protein-coding region of a gene, FXS is caused by a trinucleotide expansion in the 5′-untranslated region of the FMR1 gene, leading to hypermethylation and transcriptional silencing. Modeling FXS with human pluripotent stem cells offers a clinically relevant platform to study disease mechanisms and explore potential therapies through reactivating FMR1 expression by genetic and epigenetic means or through drug screening. This Perspective reviews the various cellular models and therapeutic strategies proposed over the past decade, highlighting their potential to advance the treatment of FXS. We also discuss the benefits and challenges of gene activation therapies, drawing comparisons with other gene-silencing disorders, including imprinting diseases and X-linked disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ng, S. B. et al. Exome sequencing identifies the cause of a Mendelian disorder. Nat. Genet. 42, 30–35 (2010).
Zhang, F. & Lupski, J. R. Non-coding genetic variants in human disease. Hum. Mol. Genet. 24, R102–R110 (2015).
Zoghbi, H. Y. & Beaudet, A. L. Epigenetics and human disease. Cold Spring Harb. Perspect. Biol. 8, a019497 (2016).
Malik, I., Kelley, C. P., Wang, E. T. & Todd, P. K. Molecular mechanisms underlying nucleotide repeat expansion disorders. Nat. Rev. Mol. Cell Biol. 22, 589–607 (2021).
Eggermann, T. et al. Imprinting disorders. Nat. Rev. Dis. Primers 9, 33 (2023).
Tabolacci, E. & Chiurazzi, P. Epigenetics, fragile X syndrome and transcriptional therapy. Am. J. Med. Genet. A 161A, 2797–2808 (2013).
Hagerman, R. J. et al. Fragile X syndrome. Nat. Rev. Dis. Primers 3, 17065 (2017).
Coffee, B. et al. Incidence of fragile X syndrome by newborn screening for methylated FMR1 DNA. Am. J. Hum. Genet. 85, 503–514 (2009).
Richter, J. D. & Zhao, X. The molecular biology of FMRP: new insights into fragile X syndrome. Nat. Rev. Neurosci. 22, 209–222 (2021).
Dahlhaus, R. Of men and mice: modeling the fragile X syndrome. Front. Mol. Neurosci. 11, 41 (2018).
Vershkov, D. & Benvenisty, N. Human pluripotent stem cells in modeling human disorders: the case of fragile X syndrome. Regen. Med. 12, 53–68 (2017).
Kumari, D. et al. Identification of fragile X syndrome-specific molecular markers in human fibroblasts: a useful model to test the efficacy of therapeutic drugs. Hum. Mutat. 35, 1485–1494 (2014).
Brasa, S. et al. Reciprocal changes in DNA methylation and hydroxymethylation and a broad repressive epigenetic switch characterize FMR1 transcriptional silencing in fragile X syndrome. Clin. Epigenetics 8, 15 (2016).
Schwartz, P. H. et al. Neural progenitor cells from an adult patient with fragile X syndrome. BMC Med. Genet. 6, 2 (2005).
Bhattacharyya, A. et al. Normal neurogenesis but abnormal gene expression in human fragile X cortical progenitor cells. Stem Cells Dev. 17, 107–117 (2008).
Castrén, M. et al. Altered differentiation of neural stem cells in fragile X syndrome. Proc. Natl Acad. Sci. USA 102, 17834–17839 (2005).
Obernier, K. & Alvarez-Buylla, A. Neural stem cells: origin, heterogeneity and regulation in the adult mammalian brain. Development 146, dev156059 (2019).
Bar, S. & Benvenisty, N. Human pluripotent stem cells: derivation and applications. Nat. Rev. Mol. Cell Biol. https://doi.org/10.1038/S41580-020-00309-7 (2020).
Bhattacharyya, A. & Zhao, X. Human pluripotent stem cell models of fragile X syndrome. Mol. Cell. Neurosci. 73, 43–51 (2015).
Mor-Shaked, H. & Eiges, R. Modeling fragile X syndrome using human pluripotent stem cells. Genes 7, 77 (2016).
Vershkov, D., Ben-Hur, T. & Benvenisty, N. in Fragile X Syndrome: from Genetics to Targeted Treatment (eds Willemsen, R. & Kooy, R. F.) 103–121 (Academic, 2017).
Burton, A. & Torres-Padilla, M. E. Epigenome dynamics in early mammalian embryogenesis. Nat. Rev. Genet. https://doi.org/10.1038/s41576-025-00831-4 (2025).
Eiges, R. et al. Developmental study of fragile X syndrome using human embryonic stem cells derived from preimplantation genetically diagnosed embryos. Cell Stem Cell 1, 568–577 (2007).
Urbach, A., Bar-Nur, O., Daley, G. Q. & Benvenisty, N. Differential modeling of fragile X syndrome by human embryonic stem cells and induced pluripotent stem cells. Cell Stem Cell 6, 407–411 (2010).
Bar-Nur, O., Russ, H. A., Efrat, S. & Benvenisty, N. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell 9, 17–23 (2011).
Hu, B. Y. et al. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc. Natl Acad. Sci. USA 107, 4335–4340 (2010).
Halevy, T., Czech, C. & Benvenisty, N. Molecular mechanisms regulating the defects in fragile X syndrome neurons derived from human pluripotent stem cells. Stem Cell Reports 4, 37–46 (2015).
Kang, Y. et al. A human forebrain organoid model of fragile X syndrome exhibits altered neurogenesis and highlights new treatment strategies. Nat. Neurosci. 24, 1377–1391 (2021).
Lee, A., Xu, J., Wen, Z. & Jin, P. Across dimensions: developing 2D and 3D human iPSC-based models of fragile X syndrome. Cells 11, 1725 (2022).
Gunapala, K. M. et al. Ascorbic acid ameliorates molecular and developmental defects in human-induced pluripotent stem cell and cerebral organoid models of fragile X syndrome. Int. J. Mol. Sci. 25, 12718 (2024).
Huang, Y. et al. Research progress, challenges, and breakthroughs of organoids as disease models. Front. Cell Dev. Biol. 9, 740574 (2021).
Shivram, H., Cress, B. F., Knott, G. J. & Doudna, J. A. Controlling and enhancing CRISPR systems. Nat. Chem. Biol. 17, 10–19 (2020).
Park, C. Y. et al. Reversion of FMR1 methylation and silencing by editing the triplet repeats in fragile X iPSC-derived neurons. Cell Rep. 13, 234–241 (2015).
Xie, N. et al. Reactivation of FMR1 by CRISPR/Cas9-mediated deletion of the expanded CGG-repeat of the fragile X chromosome. PLoS ONE 11, e0165499 (2016).
Fischer, L. A., Khan, S. A. & Theunissen, T. W. Induction of human naïve pluripotency using 5i/L/A medium. Methods Mol. Biol. 2416, 13–28 (2022).
Lee, H. G. et al. Site-specific R-loops induce CGG repeat contraction and fragile X gene reactivation. Cell 186, 2593–2609 (2023).
Williams, K., Christensen, J. & Helin, K. DNA methylation: TET proteins—guardians of CpG islands? EMBO Rep. 13, 28–35 (2012).
Groh, M. & Gromak, N. Out of balance: R-loops in human disease. PLoS Genet. 10, e1004630 (2014).
Smeets, H. J. M. et al. Normal phenotype in two brothers with a full FMR1 mutation. Hum. Mol. Genet. 4, 2103–2108 (1995).
Liu, X. S. et al. Rescue of fragile X syndrome neurons by DNA methylation editing of the FMR1 gene. Cell 172, 979–992 (2018).
Haenfler, J. M. et al. Targeted reactivation of FMR1 transcription in fragile X syndrome embryonic stem cells. Front. Mol. Neurosci. 11, 282 (2018).
Tseng, E., Tang, H. T., AlOlaby, R. R., Hickey, L. & Tassone, F. Altered expression of the FMR1 splicing variants landscape in premutation carriers. Biochim Biophys. Acta Gene Regul. Mech. 1860, 1117–1126 (2017).
Shah, S. et al. Antisense oligonucleotide rescue of CGG expansion dependent FMR1 mis-splicing in fragile X syndrome restores FMRP. Proc. Natl Acad. Sci. USA 120, e2302534120 (2023).
Colak, D. et al. Promoter-bound trinucleotide repeat mRNA drives epigenetic silencing in fragile X syndrome. Science 343, 1002–1005 (2014).
Paluszkiewicz, S. M., Martin, B. S. & Huntsman, M. M. Fragile X syndrome: the GABAergic system and circuit dysfunction. Dev. Neurosci. 33, 349–364 (2011).
Berry-Kravis, E. et al. Mavoglurant in fragile X syndrome: results of two randomized, double-blind, placebo-controlled trials. Sci. Transl. Med. 8, 321ra325 (2016).
Grabb, M. C. & Potter, W. Z. CNS trial failures: using the fragile X syndrome-mGluR5 drug target to highlight the complexities of translating preclinical discoveries into human trials. J. Clin. Psychopharmacol. 42, 234–237 (2022).
Kurosaki, T. et al. Loss of the fragile X syndrome protein FMRP results in misregulation of nonsense-mediated mRNA decay. Nat. Cell Biol. 23, 40–48 (2021).
Kumari, D. et al. High-throughput screening to identify compounds that increase fragile X mental retardation protein expression in neural stem cells differentiated from fragile X syndrome patient-derived induced pluripotent stem cells. Stem Cells Transl. Med. 4, 800–808 (2015).
Vershkov, D. et al. FMR1 reactivating treatments in fragile X iPSC-derived neural progenitors in vitro and in vivo. Cell Rep. 26, 2531–2539 (2019).
Hunt, J. F. V. et al. High throughput small molecule screen for reactivation of FMR1 in fragile X syndrome human neural cells. Cells 11, 69 (2022).
Hagemann, S., Heil, O., Lyko, F. & Brueckner, B. Azacytidine and decitabine induce gene-specific and non-random DNA demethylation in human cancer cell lines. PLoS ONE 6, e17388 (2011).
Thota, S., Oganesian, A., Azab, M. & Griffiths, E. A. Role of cedazuridine/decitabine in the management of myelodysplastic syndrome and chronic myelomonocytic leukemia. Future Oncol. 17, 2077–2087 (2021).
Vershkov, D., Yilmaz, A., Yanuka, O., Nielsen, A. L. & Benvenisty, N. Genome-wide screening for genes involved in the epigenetic basis of fragile X syndrome. Stem Cell Reports 17, 1048–1058 (2022).
Mulley, J. C. et al. FRAXE and mental retardation. J. Med. Genet. 32, 162–169 (1995).
Gecz, J., Gedeon, A. K., Sutherland, G. R. & Mulley, J. C. Identification of the gene FMR2, associated with FRAXE mental retardation. Nat. Genet. 13, 105–108 (1996).
Youings, S. A. et al. FRAXA and FRAXE: the results of a five-year survey. J. Med. Genet. 37, 415–421 (2000).
Schulz, J. B. et al. Diagnosis and treatment of Friedreich ataxia: a European perspective. Nat. Rev. Neurol. 5, 222–234 (2009).
Mosbach, V. & Puccio, H. A multiple animal and cellular models approach to study frataxin deficiency in Friedreich ataxia. Biochim. Biophys. Acta Mol. Cell Res. 1871, 119809 (2024).
Schreiber, A. M., Li, Y., Chen, Y. H., Napierala, J. S. & Napierala, M. Selected histone deacetylase inhibitors reverse the Frataxin transcriptional defect in a novel Friedreich’s ataxia induced pluripotent stem cell-derived neuronal reporter system. Front. Neurosci. 16, 836476 (2022).
Lyst, M. J. & Bird, A. Rett syndrome: a complex disorder with simple roots. Nat. Rev. Genet. 16, 261–274 (2015).
Gold, W. A. et al. Rett syndrome. Nat. Rev. Dis. Primers 10, 84 (2024).
Qian, J. et al. Multiplex epigenome editing of MECP2 to rescue Rett syndrome neurons. Sci. Transl. Med. 15, eadd4666 (2023).
Pescatore, A., Esposito, E., Draber, P., Walczak, H. & Ursini, M. V. NEMO regulates a cell death switch in TNF signaling by inhibiting recruitment of RIPK3 to the cell death-inducing complex II. Cell Death Dis. 7, e2346 (2016).
Parrish, J. E., Scheuerle, A. E., Lewis, R. A., Levy, M. L. & Nelson, D. L. Selection against mutant alleles in blood leukocytes is a consistent feature in incontinentia pigmenti type 2. Hum. Mol. Genet. 5, 1777–1783 (1996).
Kenwrick, S. et al. Survival of male patients with incontinentia pigmenti carrying a lethal mutation can be explained by somatic mosaicism or Klinefelter syndrome. Am. J. Hum. Genet. 69, 1210–1217 (2001).
Fusco, F., Fimiani, G., Tadini, G., Michele, D. & Ursini, M. V. Clinical diagnosis of incontinentia pigmenti in a cohort of male patients. J. Am. Acad. Dermatol. 56, 264–267 (2007).
Reik, W. & Walter, J. Genomic imprinting: parental influence on the genome. Nat. Rev. Genet. 2, 21–32 (2001).
Buiting, K., Williams, C. & Horsthemke, B. Angelman syndrome — insights into a rare neurogenetic disorder. Nat. Rev. Neurol. 12, 584–593 (2016).
Wakeling, E. L. Silver–Russell syndrome. Arch. Dis. Child. 96, 1156–1161 (2011).
Cassidy, S. B., Schwartz, S., Miller, J. L. & Driscoll, D. J. Prader–Willi syndrome. Genet. Med. 14, 10–26 (2012).
Docherty, L. E. et al. Clinical presentation of 6q24 transient neonatal diabetes mellitus (6q24 TNDM) and genotype–phenotype correlation in an international cohort of patients. Diabetologia 56, 758–762 (2013).
Choufani, S., Shuman, C. & Weksberg, R. Beckwith–Wiedemann syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 154C, 343–354 (2010).
Huang, H. S. et al. Topoisomerase inhibitors unsilence the dormant allele of Ube3a in neurons. Nature 481, 185–191 (2011).
Vihma, H. et al. Ube3a unsilencer for the potential treatment of Angelman syndrome. Nat. Commun. 15, 5558 (2024).
Wolter, J. M. et al. Cas9 gene therapy for Angelman syndrome traps Ube3a-ATS long non-coding RNA. Nature 587, 281–284 (2020).
Schmid, R. S. et al. CRISPR/Cas9 directed to the Ube3a antisense transcript improves Angelman syndrome phenotype in mice. J. Clin. Invest. 131, e142574 (2021).
Rohm, D. et al. Activation of the imprinted Prader–Willi syndrome locus by CRISPR-based epigenome editing. Cell Genom. 5, 100770 (2025).
Wang, S. E. & Jiang, Y.-H. Novel epigenetic molecular therapies for imprinting disorders. Mol. Psychiatry 28, 3182–3193 (2023).
Garber, K. B., Visootsak, J. & Warren, S. T. Fragile X syndrome. Eur. J. Hum. Genet. 16, 666–672 (2008).
Rodriguez-Revenga, L. et al. Penetrance of FMR1 premutation associated pathologies in fragile X syndrome families. Eur. J. Hum. Genet. 17, 1359–1362 (2009).
Tassone, F. et al. Elevated levels of FMR1 mRNA carrier males: a new mechanism of involvement in the fragile-X syndrome. Am. J. Hum. Genet. 66, 6–15 (2000).
Tassone, F. et al. Elevated FMR1 mRNA in premutation carriers is due to increased transcription. RNA 13, 555–562 (2007).
Kraan, C. M., Godler, D. E. & Amor, D. J. Epigenetics of fragile X syndrome and fragile X-related disorders. Dev. Med. Child Neurol. 61, 121–127 (2019).
Landy, S. J. & Donnai, D. Incontinentia pigmenti (Bloch–Sulzberger syndrome). J. Med. Genet. 30, 53–59 (1993).
Vaghani, U. P. et al. Bloch–Sulzberger syndrome: a rare X-linked dominant genetic disorder in a newborn. Cureus 15, e48823 (2023).
Prasasya, R., Grotheer, K. V., Siracusa, L. D. & Bartolomei, M. S. Temple syndrome and Kagami–Ogata syndrome: clinical presentations, genotypes, models and mechanisms. Hum. Mol. Genet. 29, R107–R116 (2020).
Baena, N. et al. Novel 14q32.2 paternal deletion encompassing the whole DLK1 gene associated with Temple syndrome. Clin. Epigenetics 16, 62 (2024).
Acknowledgements
We thank all members of the Azrieli Center for Stem Cells and Genetic Research for critical reading of the manuscript. N.B. was supported by the Azrieli Foundation, the Rosetrees Trust, the Israel Science Foundation (no. 2054/22) and the ISF–Israel Precision Medicine Partnership Program (no. 3605/21). A.G is a PhD fellow of the Neubauer Foundation. N.B. is the Herbert Cohn Chair in Cancer Research.
Author information
Authors and Affiliations
Contributions
A.G., K.M.G., V.T. and N.B. conceived the main ideas presented in this Perspective. A.G. drafted the manuscript with contribution by K.M.G., V.T. and N.B. N.B. supervised the submission of the Perspective and secured funding.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Genetics thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Table 1
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gadban, A., Gunapala, K.M., Taylor, V. et al. Therapeutic strategies for fragile X syndrome and implications for other gene-silencing disorders. Nat Genet 57, 1812–1822 (2025). https://doi.org/10.1038/s41588-025-02255-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41588-025-02255-6