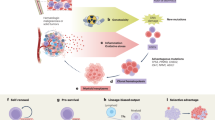
Abstract
Clonal hematopoiesis (CH) results from the acquisition and expansion of somatic mutations in hematopoietic stem and progenitor cells and is associated with age-related clinical sequelae, including an increased risk for cardiovascular disease, myeloid neoplasms and complications related to cancer therapy. Chemotherapy and radiation can accelerate CH expansion and further elevate the risk of adverse events, including cardiotoxicity and therapy-related myeloid neoplasms. Although CH is increasingly recognized as a clinically relevant precursor state and predictive biomarker, the long-term dynamics of CH expansion in humans remain poorly understood. Longitudinal data are often collected but not integrated with mathematical prediction. Mathematical modeling is essential for characterizing CH evolution, estimating clone fitness, inferring stem cell pool dynamics and enabling patient-level predictions. This study summarizes the current evidence on CH dynamics in humans, compares mathematical models used to predict CH progression, assesses the validity of model assumptions and discusses the implications for clinical management of individuals with these precursor conditions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bowman, R. L., Busque, L. & Levine, R. L. Clonal hematopoiesis and evolution to hematopoietic malignancies. Cell Stem Cell 22, 157–170 (2018).
Watson, C. J. et al. The evolutionary dynamics and fitness landscape of clonal hematopoiesis. Science 367, 1449–1454 (2020).
Fabre, M. A. et al. The longitudinal dynamics and natural history of clonal haematopoiesis. Nature 606, 335–342 (2022).
Williams, N. et al. Life histories of myeloproliferative neoplasms inferred from phylogenies. Nature 602, 162–168 (2022).
Weeks, L. D. & Ebert, B. L. Causes and consequences of clonal hematopoiesis. Blood 142, 2235–2246 (2023).
Stiehl, T. Stem cell graft dose and composition could impact on the expansion of donor-derived clones after allogeneic hematopoietic stem cell transplantation—a virtual clinical trial. Front. Immunol. 15, 1321336 (2024).
Abegunde, S. O., Buckstein, R., Wells, R. A. & Rauh, M. J. An inflammatory environment containing TNFα favors Tet2-mutant clonal hematopoiesis. Exp. Hematol. 59, 60–65 (2018).
Hormaechea-Agulla, D. et al. Chronic infection drives Dnmt3a-loss-of-function clonal hematopoiesis via IFNγ signaling. Cell Stem Cell 28, 1428–1442 (2021).
Caiado, F. et al. Aging drives Tet2+/− clonal hematopoiesis via IL-1 signaling. Blood 141, 886–903 (2023) .
Weeks, L. D. et al. Prediction of risk for myeloid malignancy in clonal hematopoiesis. NEJM Evid. 2, https://doi.org/10.1056/evidoa2200310 (2023).
Arber, D. A. et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127, 2391–2405 (2016).
Jaiswal, S. & Ebert, B. L. Clonal hematopoiesis in human aging and disease. Science 366, eaan4673 (2019).
Malcovati, L. et al. Clinical significance of somatic mutation in unexplained blood cytopenia. Blood 129, 3371–3378 (2017).
Song, J. et al. Co-mutation of ASXL1 and SF3B1 predicts poorer overall survival than isolated ASXL1 or SF3B1 mutations. In Vivo 37, 985–993 (2023).
Bejar, R. et al. Clinical effect of point mutations in myelodysplastic syndromes. N. Engl. J. Med. 364, 2496–2506 (2011).
Deininger, M. W. N., Tyner, J. W. & Solary, E. Turning the tide in myelodysplastic/myeloproliferative neoplasms. Nat. Rev. Cancer 17, 425–440 (2017).
Williams, M. J. et al. Measuring the distribution of fitness effects in somatic evolution by combining clonal dynamics with dN/dS ratios. eLife 9, e48714 (2020).
Loh, P.-R. et al. Insights into clonal haematopoiesis from 8,342 mosaic chromosomal alterations. Nature 559, 350–355 (2018).
Heyde, A. et al. Increased stem cell proliferation in atherosclerosis accelerates clonal hematopoiesis. Cell 184, 1348–1361 (2021).
Huillet, T. & Möhle, M. On the extended Moran model and its relation to coalescents with multiple collisions. Theor. Popul. Biol. 87, 5–14 (2013).
West, J., Robertson-Tessi, M. & Anderson, A. R. A. Agent-based methods facilitate integrative science in cancer. Trends Cell Biol. 33, 300–311 (2023).
Mitchell, E. et al. Clonal dynamics of haematopoiesis across the human lifespan. Nature 606, 343–350 (2022).
Welch, J. S. et al. The origin and evolution of mutations in acute myeloid leukemia. Cell 150, 264–278 (2012).
Kreger, J., Mooney, J. A., Shibata, D. & MacLean, A. L. Developmental hematopoietic stem cell variation explains clonal hematopoiesis later in life. Nat. Commun. 15, 10268 (2024).
Catlin, S. N., Busque, L., Gale, R. E., Guttorp, P. & Abkowitz, J. L. The replication rate of human hematopoietic stem cells in vivo. Blood 117, 4460–4466 (2011).
Johnson, B., Shuai, Y., Schweinsberg, J. & Curtius, K. cloneRate: fast estimation of single-cell clonal dynamics using coalescent theory. Bioinformatics 39, btad561 (2023).
Bernstein, N. et al. Analysis of somatic mutations in whole blood from 200,618 individuals identifies pervasive positive selection and novel drivers of clonal hematopoiesis. Nat. Genet. 56, 1147–1155 (2024).
Uryu, H. et al. Clonal evolution of hematopoietic stem cells after autologous stem cell transplantation. Nat. Genet. 57, 1695–1707 (2025).
Zink, F. et al. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood 130, 742–752 (2017).
Abelson, S. et al. Prediction of acute myeloid leukaemia risk in healthy individuals. Nature 559, 400–404 (2018).
Rubinow, S. I. & Lebowitz, J. L. A mathematical model of neutrophil production and control in normal man. J. Math. Biol. 1, 187–225 (1975).
Rodriguez-Brenes, I. A., Wodarz, D. & Komarova, N. L. Minimizing the risk of cancer: tissue architecture and cellular replication limits. J. R. Soc. Interface 10, 20130410 (2013).
Gravenmier, C. et al. Cell state transitions drive the evolution of disease progression in B-lymphoblastic leukemia. Cancer Res. Commun. 6, 47–59 (2026).
Jamieson, C. H. M. et al. Granulocyte–macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N. Engl. J. Med. 351, 657–667 (2004).
Mackey, M. C. & Dörmer, P. Continuous maturation of proliferating erythroid precursors. Cell Tissue Kinet. 15, 381–392 (1982).
Haurie, C., Dale, D. C. & Mackey, M. C. Cyclical neutropenia and other periodic hematological disorders: a review of mechanisms and mathematical models. Blood 92, 2629–2640 (1998).
Velten, L. et al. Human haematopoietic stem cell lineage commitment is a continuous process. Nat. Cell Biol. 19, 271–281 (2017).
Zhang, Y., Stiehl, T. & Tang, M. A continuous integral model for white blood cell production. SIAM J. Appl. Math. 82, 2111–2130 (2022).
Cho, H. et al. Modelling acute myeloid leukaemia in a continuum of differentiation states. Lett. Biomath. 5, S69–S98 (2018).
Marciniak-Czochra, A., Stiehl, T., Ho, A. D., Jger, W. & Wagner, W. Modeling of asymmetric cell division in hematopoietic stem cells—regulation of self-renewal is essential for efficient repopulation. Stem Cells Dev. 18, 377–386 (2009).
Arino, O. & Kimmel, M. Stability analysis of models of cell production systems. Math. Model. 7, 1269–1300 (1986).
Komarova, N. L., Rignot, C., Fleischman, A. G. & Wodarz, D. Dynamically adjusted cell fate decisions and resilience to mutant invasion during steady-state hematopoiesis revealed by an experimentally parameterized mathematical model. Proc. Natl Acad. Sci. USA 121, e2321525121 (2024).
Stiehl, T. & Marciniak-Czochra, A. Characterization of stem cells using mathematical models of multistage cell lineages. Math. Comput. Model. 53, 1505–1517 (2011).
Kumar, R., Shah, S. R. & Stiehl, T. Understanding the impact of feedback regulations on blood cell production and leukemia dynamics using model analysis and simulation of clinically relevant scenarios. Appl. Math. Model. 129, 340–389 (2024).
Komarova, N. L. Principles of regulation of self-renewing cell lineages. PLoS ONE 8, e72847 (2013).
Mon Père, N. V., Lenaerts, T., Pacheco, J. M. D. S. & Dingli, D. Multistage feedback-driven compartmental dynamics of hematopoiesis. iScience 24, 102326 (2021).
Stiehl, T., Wang, W., Lutz, C. & Marciniak-Czochra, A. Mathematical modeling provides evidence for niche competition in human AML and serves as a tool to improve risk stratification. Cancer Res. 80, 3983–3992 (2020).
Stiehl, T., Baran, N., Ho, A. D. & Marciniak-Czochra, A. Cell division patterns in acute myeloid leukemia stem-like cells determine clinical course: a model to predict patient survival. Cancer Res. 75, 940–949 (2015).
Stiehl, T., Lutz, C. & Marciniak-Czochra, A. Emergence of heterogeneity in acute leukemias. Biol. Direct. 11, 51 (2016).
Ahmed, I., Dingli, D., Huang, W. & Werner, B. Clonal fitness decline in somatic differentiation hierarchies. Preprint at bioRxiv https://doi.org/10.1101/2023.09.27.559763 (2023).
Buck, M. C. et al. Progressive disruption of hematopoietic architecture from clonal hematopoiesis to MDS. iScience 26, 107328 (2023).
Walenda, T. et al. Feedback signals in myelodysplastic syndromes: increased self-renewal of the malignant clone suppresses normal hematopoiesis. PLoS Comput. Biol. 10, e1003599 (2014).
Jaiswal, S. et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 371, 2488–2498 (2014).
Genovese, G. et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N. Engl. J. Med. 371, 2477–2487 (2014).
Young, A. L., Challen, G. A., Birmann, B. M. & Druley, T. E. Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat. Commun. 7, 12484 (2016).
Père, N. V. M., Terenzi, F. & Werner, B. The dynamic fitness landscape of ageing haematopoiesis through clonal competition. Preprint at bioRxiv https://doi.org/10.1101/2024.04.16.589764 (2024).
Franceschi, C. et al. Inflamm-aging: an evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 908, 244–254 (2000).
Colotta, F., Allavena, P., Sica, A., Garlanda, C. & Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 30, 1073–1081 (2009).
Ben-Crentsil, N. A. et al. RNA shielding of p65 is required to potentiate oncogenic inflammation in TET2-mutated clonal hematopoiesis. Cancer Discov. 14, 2509–2531 (2024).
Pasupuleti, S. K. et al. Obesity induced inflammation exacerbates clonal hematopoiesis. J. Clin. Investig. 133, e163968 (2023).
Sano, S. et al. Tet2-mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the IL-1β/NLRP3 inflammasome. J. Am. Coll. Cardiol. 71, 875–886 (2018).
Asada, S. & Kitamura, T. Clonal hematopoiesis and associated diseases: a review of recent findings. Cancer Sci. 112, 3962–3971 (2021).
Cook, E. K., Luo, M. & Rauh, M. J. Clonal hematopoiesis and inflammation: partners in leukemogenesis and comorbidity. Exp. Hematol. 83, 85–94 (2020).
Yu, Z. et al. Genetic modification of inflammation and clonal hematopoiesis-associated cardiovascular risk. J. Clin. Invest. 133, e168597 (2023).
Bick, A. G. et al. Genetic IL-6 signaling deficiency attenuates cardiovascular risk in clonal hematopoiesis. Circulation 141, 124–131 (2019).
Larsen, M. K. et al. Clonal haematopoiesis of indeterminate potential and impaired kidney function—a Danish general population study with 11 years follow-up. Eur. J. Haematol. 109, 576–585 (2022).
Bolton, K. L. et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat. Genet. 52, 1219–1226 (2020).
Mitchell, E. et al. The long-term effects of chemotherapy on normal blood cells. Nat. Genet. 57, 1684–1694 (2025).
Ottesen, J. T. & Andersen, M. Potential of immunotherapies in treating hematological cancer-infection comorbidities—a mathematical modelling approach. Cancers (Basel) 13, 3789 (2021).
Ottesen, J. T. & Andersen, M. Aging, inflammation, and comorbidity in cancers—a general in silico study exemplified by myeloproliferative malignancies. Cancers (Basel) 15, 4806 (2023).
Stiehl, T., Ho, A. D. & Marciniak-Czochra, A. Mathematical modeling of the impact of cytokine response of acute myeloid leukemia cells on patient prognosis. Sci. Rep. 8, 2809 (2018).
Pedersen, R. K. et al. HSC niche dynamics in regeneration, pre-malignancy, and cancer: insights from mathematical modeling. Stem Cells 41, 260–270 (2023).
Andersen, M. et al. Mathematical modelling as a proof of concept for MPNs as a human inflammation model for cancer development. PLoS ONE 12, e0183620 (2017).
Andersen, M., Hasselbalch, H. C., Kjær, L., Skov, V. & Ottesen, J. T. Global dynamics of healthy and cancer cells competing in the hematopoietic system. Math. Biosci. 326, 108372 (2020).
Ottesen, J. T. et al. Bridging blood cancers and inflammation: the reduced Cancitis model. J. Theor. Biol. 465, 90–108 (2019).
Sajid, Z., Andersen, M. & Ottesen, J. T. Mathematical analysis of the Cancitis model and the role of inflammation in blood cancer progression. Math. Biosci. Eng. 16, 8268–8289 (2019).
Ottesen, J. T. et al. Mathematical modeling of MPNs offers understanding and decision support for personalized treatment. Cancers (Basel) 12, 2119 (2020).
Mosca, M. et al. Inferring the dynamics of mutated hematopoietic stem and progenitor cells induced by IFNα in myeloproliferative neoplasms. Blood 138, 2231–2243 (2021).
Boklund, T. I. et al. Mathematical modelling of stem and progenitor cell dynamics during ruxolitinib treatment of patients with myeloproliferative neoplasms. Front. Immunol. 15, 1384509 (2024).
Boettcher, S. et al. Clonal hematopoiesis in donors and long-term survivors of related allogeneic hematopoietic stem cell transplantation. Blood 135, 1548–1559 (2020).
Uddin, M. M. et al. Long-term longitudinal analysis of 4,187 participants reveals insights into determinants of clonal hematopoiesis. Nat. Commun. 15, 7858 (2024).
Van Zeventer, I. A. et al. Evolutionary landscape of clonal hematopoiesis in 3,359 individuals from the general population. Cancer Cell 41, 1017–1031 (2023).
Robertson, N. A. et al. Longitudinal dynamics of clonal hematopoiesis identifies gene-specific fitness effects. Nat. Med. 28, 1439–1446 (2022).
Larsen, M. K. et al. The JAK2V617F and CALR mutations and risk of cancer, cardiovascular diseases, and all-cause mortality. J. Intern. Med. 299, 79–94 (2026).
Gillis, N. K. et al. Clonal haemopoiesis and therapy-related myeloid malignancies in elderly patients: a proof-of-concept, case-control study. Lancet Oncol. 18, 112–121 (2017).
Gu, M. et al. Multiparameter prediction of myeloid neoplasia risk. Nat. Genet. 55, 1523–1530 (2023).
Kwok, B. et al. MDS-associated somatic mutations and clonal hematopoiesis are common in idiopathic cytopenias of undetermined significance. Blood 126, 2355–2361 (2015).
Makishima, H. et al. Dynamics of clonal evolution in myelodysplastic syndromes. Nat. Genet. 49, 204–212 (2017).
Menssen, A. J. et al. Convergent clonal evolution of signaling gene mutations is a hallmark of myelodysplastic syndrome progression. Blood Cancer Discov. 3, 330–345 (2022).
Walter, M. J. et al. Clonal architecture of secondary acute myeloid leukemia. N. Engl. J. Med. 366, 1090–1098 (2012).
Guess, T. et al. Distinct patterns of clonal evolution drive myelodysplastic syndrome progression to secondary acute myeloid leukemia. Blood Cancer Discov. 3, 316–329 (2022).
Miles, L. A. et al. Single-cell mutation analysis of clonal evolution in myeloid malignancies. Nature 587, 477–482 (2020).
Morita, K. et al. Clonal evolution of acute myeloid leukemia revealed by high-throughput single-cell genomics. Nat. Commun. 11, 5327 (2020).
Jacoby, M. A. et al. Subclones dominate at MDS progression following allogeneic hematopoietic cell transplant. JCI Insight 3, e98962 (2018).
Cai, Z. et al. Inhibition of inflammatory signaling in Tet2 mutant preleukemic cells mitigates stress-induced abnormalities and clonal mematopoiesis. Cell Stem Cell 23, 833–849 (2018).
Agarwal, P. et al. Microbial metabolite drives ageing-related clonal haematopoiesis via ALPK1. Nature 642, 201–211 (2025).
Wong, T. N. et al. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature 518, 552–555 (2015).
Christopher, M. J. et al. Immune escape of relapsed AML cells after allogeneic transplantation. N. Engl. J. Med. 379, 2330–2341 (2018).
Nangalia, J. et al. DNMT3A mutations occur early or late in patients with myeloproliferative neoplasms and mutation order influences phenotype. Haematologica 100, e438–e442 (2015).
Turajlic, S., Sottoriva, A., Graham, T. & Swanton, C. Resolving genetic heterogeneity in cancer. Nat. Rev. Genet. 20, 404–416 (2019).
Moeller, M. E., Père, N. V. M., Werner, B. & Huang, W. Measures of genetic diversification in somatic tissues at bulk and single-cell resolution. eLife 12, RP89780 (2024).
Stein, A. & Werner, B. On the patterns of genetic intra-tumor heterogeneity before and after treatment. Genetics 230, iyaf101 (2025).
Gunnarsson, E. B., Leder, K. & Foo, J. Exact site frequency spectra of neutrally evolving tumors: a transition between power laws reveals a signature of cell viability. Theor. Popul. Biol. 142, 67–90 (2021).
Durrett, R. Branching Process Models of Cancer 1–63 (Springer, 2015).
Poon, G. Y. P., Watson, C. J., Fisher, D. S. & Blundell, J. R. Synonymous mutations reveal genome-wide levels of positive selection in healthy tissues. Nat. Genet. 53, 1597–1605 (2021).
Gabbutt, C. et al. Fluctuating methylation clocks for cell lineage tracing at high temporal resolution in human tissues. Nat. Biotechnol. 40, 720–730 (2022).
Sørensen, A. L. et al. Elevated levels of oxidized nucleosides in individuals with the JAK2V617F mutation from a general population study. Redox Biol. 41, 101895 (2021).
Hasselbalch, H. C. et al. The CHIP-clinic as the catalyst of preventive medicine. Front. Hematol. 3, 1459154 (2024).
Acknowledgements
The authors gratefully acknowledge funding from Moffitt Cancer Center via the Center of Excellence for Evolutionary Therapy (to S.M. and J.W.). N.V.M.P. acknowledges support from the Barts Charity. B.W. is supported by a Barts Charity Lectureship (grant MGU045) and a UKRI Future Leaders Fellowship (grant MR/V02342X/1). T.S., J.S. and J.G.H. acknowledge support from the Lundbeck Foundation (grant R335-2019-2020).
Author information
Authors and Affiliations
Contributions
S.M. designed the figures, with help from N.V.M.P., B.W., W.H. and J.W. All authors contributed to the writing, reviewing and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Genetics thanks Moritz Gerstung and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marzban, S., Stiehl, T., Xie, Z. et al. Modeling the evolutionary dynamics of clonal hematopoiesis. Nat Genet (2026). https://doi.org/10.1038/s41588-026-02504-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41588-026-02504-2