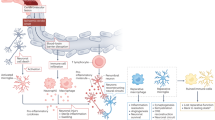
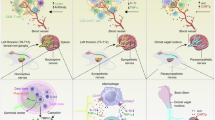
Abstract
Injury is a key driver of inflammation, a critical yet necessary response involving several mediators that is aimed at restoring tissue homeostasis. Inflammation in the central nervous system can be triggered by a variety of stimuli, some intrinsic to the brain and others arising from peripheral signals. Fine-tuned regulation of this response is crucial in a system that is vulnerable due to, for example, aging and ongoing neurodegeneration. In this context, seemingly harmless interventions like a common surgery to repair a broken limb can overwhelm the immune system and become the driver of further complications such as delirium and other perioperative neurocognitive disorders. Here, we discuss potential mechanisms by which the immune system affects the central nervous system after surgical trauma. Together, these neuroimmune interactions are becoming hallmarks of and potential therapeutic targets for multiple neurologic conditions, including those affecting the perioperative space.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Pluvinage, J. V. & Wyss-Coray, T. Systemic factors as mediators of brain homeostasis, ageing and neurodegeneration. Nat. Rev. Neurosci. 21, 93–102 (2020).
Soares, H. D., Hicks, R. R., Smith, D. & McIntosh, T. K. Inflammatory leukocytic recruitment and diffuse neuronal degeneration are separate pathological processes resulting from traumatic brain injury. J. Neurosci. 15, 8223–8233 (1995).
Gelderblom, M. et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 40, 1849–1857 (2009).
Evered, L. & Eckenhoff, R. G. & International Perioperative Cognition Nomenclature Working Group. Perioperative cognitive disorders. Response to: postoperative delirium portends descent to dementia. Br. J. Anaesth. 119, 1241 (2017).
Evered, L. A. & Silbert, B. S. Postoperative cognitive dysfunction and noncardiac surgery. Anesth. Analg. 127, 496–505 (2018).
Rasmussen, L. S. et al. Does anaesthesia cause postoperative cognitive dysfunction? A randomised study of regional versus general anaesthesia in 438 elderly patients. Acta Anaesthesiol. Scand. 47, 260–266 (2003).
Inouye, S. K., Westendorp, R. G. J. & Saczynski, J. S. Delirium in elderly people. Lancet 383, 911–922 (2014).
Koster, S., Hensens, A. G., Schuurmans, M. J. & van der Palen, J. Consequences of delirium after cardiac operations. Ann. Thorac. Surg. 93, 705–711 (2012).
Robinson, T. N. et al. Postoperative delirium in the elderly: risk factors and outcomes. Ann. Surg. 249, 173–178 (2009).
Marcantonio, E. R., Flacker, J. M., Michaels, M. & Resnick, N. M. Delirium is independently associated with poor functional recovery after hip fracture. J. Am. Geriatr. Soc. 48, 618–624 (2000).
Inouye, S. K. et al. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement. 12, 766–775 (2016).
Saczynski, J. S. et al. Cognitive trajectories after postoperative delirium. N. Engl. J. Med. 367, 30–39 (2012).
Davis, D. H. J. et al. Association of delirium with cognitive decline in late life: a neuropathologic study of 3 population-based cohort studies. JAMA Psychiatry 74, 244–251 (2017).
Fong, T. G. et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology 72, 1570–1575 (2009).
Prince, M. et al. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 9, 63–75.e2 (2013).
Fick, D. M., Agostini, J. V. & Inouye, S. K. Delirium superimposed on dementia: a systematic review. J. Am. Geriatr. Soc. 50, 1723–1732 (2002).
Lee, H. B., Oldham, M. A., Sieber, F. E. & Oh, E. S. Impact of delirium after hip fracture surgery on one-year mortality in patients with or without dementia: a case of effect modification. Am. J. Geriatr. Psychiatry 25, 308–315 (2017).
AGS/NIA Delirium Conference Writing Group, Planning Committee and Faculty. The American Geriatrics Society/National Institute on Aging Bedside-to-Bench Conference: research agenda on delirium in older adults. J. Am. Geriatr. Soc. 63, 843–852 (2015).
Kotfis, K. et al. COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit. Care 24, 176 (2020).
Leslie, D. L., Marcantonio, E. R., Zhang, Y., Leo-Summers, L. & Inouye, S. K. One-year health care costs associated with delirium in the elderly. Arch. Intern. Med. 168, 27–32 (2008).
Fong, T. G., Davis, D., Growdon, M. E., Albuquerque, A. & Inouye, S. K. The interface between delirium and dementia in elderly adults. Lancet Neurol. 14, 823–832 (2015).
Louveau, A., Harris, T. H. & Kipnis, J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol. 36, 569–577 (2015).
Sweeney, M. D., Sagare, A. P. & Zlokovic, B. V. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 14, 133–150 (2018).
Zindel, J. & Kubes, P. DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Annu. Rev. Pathol. 15, 493–518 (2020).
Huber-Lang, M., Lambris, J. D. & Ward, P. A. Innate immune responses to trauma. Nat. Immunol. 19, 327–341 (2018).
Lotze, M. T. & Tracey, K. J. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 5, 331–342 (2005).
Scaffidi, P., Misteli, T. & Bianchi, M. E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418, 191–195 (2002).
Terrando, N. et al. Tumor necrosis factor-α triggers a cytokine cascade yielding postoperative cognitive decline. Proc. Natl Acad. Sci. USA 107, 20518–20522 (2010).
Vacas, S., Degos, V., Tracey, K. J. & Maze, M. High-mobility group box 1 protein initiates postoperative cognitive decline by engaging bone marrow–derived macrophages. Anesthesiology 120, 1160–1167 (2014).
Venereau, E. et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J. Exp. Med. 209, 1519–1528 (2012).
Lu, S.-M. et al. S100A8 contributes to postoperative cognitive dysfunction in mice undergoing tibial fracture surgery by activating the TLR4/MyD88 pathway. Brain Behav. Immun. 44, 221–234 (2015).
Fonken, L. K. et al. The alarmin HMGB1 mediates age-induced neuroinflammatory priming. J. Neurosci. 36, 7946–7956 (2016).
Terrando, N. et al. Systemic HMGB1 neutralization prevents postoperative neurocognitive dysfunction in aged rats. Front. Immunol. 7, 441 (2016).
Lamkanfi, M. et al. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J. Immunol. 185, 4385–4392 (2010).
Cape, E. et al. Cerebrospinal fluid markers of neuroinflammation in delirium: a role for interleukin-1β in delirium after hip fracture. J. Psychosom. Res. 77, 219–225 (2014).
Cibelli, M. et al. Role of interleukin-1β in postoperative cognitive dysfunction. Ann. Neurol. 68, 360–368 (2010).
Zheng, B., Lai, R., Li, J. & Zuo, Z. Critical role of P2X7 receptors in the neuroinflammation and cognitive dysfunction after surgery. Brain Behav. Immun. 61, 365–374 (2017).
Ji, S.-R., Wu, Y., Potempa, L. A., Liang, Y.-H. & Zhao, J. Effect of modified C-reactive protein on complement activation: a possible complement regulatory role of modified or monomeric C-reactive protein in atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 26, 935–941 (2006).
Sjöberg, A. P., Trouw, L. A., McGrath, F. D. G., Hack, C. E. & Blom, A. M. Regulation of complement activation by C-reactive protein: targeting of the inhibitory activity of C4b-binding protein. J. Immunol. 176, 7612–7620 (2006).
Bíro, A. et al. Studies on the interactions between C-reactive protein and complement proteins. Immunology 121, 40–50 (2007).
Suresh, M. V., Singh, S. K., Ferguson, D. A. Jr. & Agrawal, A. Role of the property of C-reactive protein to activate the classical pathway of complement in protecting mice from pneumococcal infection. J. Immunol. 176, 4369–4374 (2006).
Hecke, F. et al. Circulating complement proteins in multiple trauma patients—correlation with injury severity, development of sepsis, and outcome. Crit. Care Med. 25, 2015–2024 (1997).
Thordardottir, S., Vikingsdottir, T., Bjarnadottir, H., Jonsson, H. Jr. & Gudbjornsson, B. Activation of complement following total hip replacement. Scand. J. Immunol. 83, 219–224 (2016).
Hoedemaekers, C. et al. The complement system is activated in a biphasic pattern after coronary artery bypass grafting. Ann. Thorac. Surg. 89, 710–716 (2010).
Xiong, C. et al. Complement activation contributes to perioperative neurocognitive disorders in mice. J. Neuroinflammation 15, 254 (2018).
Shi, Q. et al. Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP/PS1 mice. Sci. Transl. Med. 9, eaaf6295 (2017).
Alawieh, A., Langley, E. F. & Tomlinson, S. Targeted complement inhibition salvages stressed neurons and inhibits neuroinflammation after stroke in mice. Sci. Transl. Med. 10, eaao6459 (2018).
Westhoff, D. et al. Preoperative protein profiles in cerebrospinal fluid in elderly hip fracture patients at risk for delirium: a proteomics and validation study. BBA Clin. 4, 115–122 (2015).
Reis, E. S., Mastellos, D. C., Hajishengallis, G. & Lambris, J. D. New insights into the immune functions of complement. Nat. Rev. Immunol. 19, 503–516 (2019).
Adams, R. A. et al. The fibrin-derived γ377-395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J. Exp. Med. 204, 571–582 (2007).
Merlini, M. et al. Fibrinogen induces microglia-mediated spine elimination and cognitive impairment in an Alzheimer’s disease model. Neuron 101, 1099–1108.e6 (2019).
Terrando, N. et al. Resolving postoperative neuroinflammation and cognitive decline. Ann. Neurol. 70, 986–995 (2011).
Takizawa, F., Tsuji, S. & Nagasawa, S. Enhancement of macrophage phagocytosis upon iC3b deposition on apoptotic cells. FEBS Lett. 397, 269–272 (1996).
Ramaglia, V. et al. C3-dependent mechanism of microglial priming relevant to multiple sclerosis. Proc. Natl Acad. Sci. USA 109, 965–970 (2012).
Velagapudi, R. et al. Orthopedic surgery triggers attention deficits in a delirium-like mouse model. Front. Immunol. 10, 2675 (2019).
Wang, P. et al. Neurovascular and immune mechanisms that regulate postoperative delirium superimposed on dementia. Alzheimers Dement. 16, 734–749 (2020).
Petersen, M. A., Ryu, J. K. & Akassoglou, K. Fibrinogen in neurological diseases: mechanisms, imaging and therapeutics. Nat. Rev. Neurosci. 19, 283–301 (2018).
McNeil, J. B. et al. Plasma biomarkers of inflammation, coagulation, and brain injury as predictors of delirium duration in older hospitalized patients. PLoS ONE 14, e0226412 (2019).
Khan, B. A. et al. Biomarkers of delirium duration and delirium severity in the ICU. Crit. Care Med. 48, 353–361 (2020).
Hov, K. R. et al. Blood-cerebrospinal fluid barrier integrity in delirium determined by Q-albumin. Dement. Geriatr. Cogn. Disord. 41, 192–198 (2016).
Hughes, C. G. et al. Endothelial activation and blood-brain barrier injury as risk factors for delirium in critically ill patients. Crit. Care Med. 44, e809–e817 (2016).
Yang, T. et al. Maresin 1 attenuates neuroinflammation in a mouse model of perioperative neurocognitive disorders. Br. J. Anaesth. 122, 350–360 (2019).
Degos, V. et al. Depletion of bone marrow–derived macrophages perturbs the innate immune response to surgery and reduces postoperative memory dysfunction. Anesthesiology 118, 527–536 (2013).
Yang, S. et al. Anesthesia and surgery impair blood–brain barrier and cognitive function in mice. Front. Immunol. 8, 902 (2017).
Deiner, S. et al. Human plasma biomarker responses to inhalational general anaesthesia without surgery. Br. J. Anaesth. 125, 282–290 (2020).
Hu, J. et al. Interleukin-6 is both necessary and sufficient to produce perioperative neurocognitive disorder in mice. Br. J. Anaesth. 120, 537–545 (2018).
Ni, P. et al. IL-17A contributes to perioperative neurocognitive disorders through blood-brain barrier disruption in aged mice. J. Neuroinflammation 15, 332 (2018).
Zhang, X. et al. Activated brain mast cells contribute to postoperative cognitive dysfunction by evoking microglia activation and neuronal apoptosis. J. Neuroinflammation 13, 127 (2016).
Rempe, R. G. et al. Matrix metalloproteinase-mediated blood-brain barrier dysfunction in epilepsy. J. Neurosci. 38, 4301–4315 (2018).
Li, Z. et al. Surgery-induced hippocampal angiotensin II elevation causes blood-brain barrier disruption via MMP/TIMP in aged rats. Front. Cell Neurosci. 10, 105 (2016).
Bi, J., Shan, W., Luo, A. & Zuo, Z. Critical role of matrix metallopeptidase 9 in postoperative cognitive dysfunction and age-dependent cognitive decline. Oncotarget 8, 51817–51829 (2017).
Senatorov, V. V. Jr. et al. Blood-brain barrier dysfunction in aging induces hyperactivation of TGFβ signaling and chronic yet reversible neural dysfunction. Sci. Transl. Med. 11, eaaw8283 (2019).
Schachtrup, C. et al. Fibrinogen triggers astrocyte scar formation by promoting the availability of active TGF-beta after vascular damage. J. Neurosci. 30, 5843–5854 (2010).
Montagne, A. et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85, 296–302 (2015).
Song, W. M. & Colonna, M. The identity and function of microglia in neurodegeneration. Nat. Immunol. 19, 1048–1058 (2018).
Forsberg, A. et al. The immune response of the human brain to abdominal surgery. Ann. Neurol. 81, 572–582 (2017).
Owen, D. R. et al. Determination of [11C]PBR28 binding potential in vivo: a first human TSPO blocking study. J. Cereb. Blood Flow Metab. 34, 989–994 (2014).
Notter, T. et al. Translational evaluation of translocator protein as a marker of neuroinflammation in schizophrenia. Mol. Psychiatry 23, 323–334 (2018).
Cosenza-Nashat, M. et al. Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol. Appl. Neurobiol. 35, 306–328 (2009).
Feng, X. et al. Microglia mediate postoperative hippocampal inflammation and cognitive decline in mice. JCI Insight 2, e91229 (2017).
Huang, Y. et al. Repopulated microglia are solely derived from the proliferation of residual microglia after acute depletion. Nat. Neurosci. 21, 530–540 (2018).
Valdearcos, M. et al. Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab. 26, 185–197.e3 (2017).
Bettcher, B. M. et al. Increases in a pro-inflammatory chemokine, MCP-1, are related to decreases in memory over time. Front. Aging Neurosci. 11, 25 (2019).
Deshmane, S. L., Kremlev, S., Amini, S. & Sawaya, B. E. Monocyte chemoattractant protein-1 (MCP-1): an overview. J. Interferon Cytokine Res. 29, 313–326 (2009).
Henjum, K. et al. CSF sTREM2 in delirium—relation to Alzheimer’s disease CSF biomarkers Aβ42, t-tau and p-tau. J. Neuroinflammation 15, 304 (2018).
Keren-Shaul, H. et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290.e17 (2017).
Linnartz-Gerlach, B. et al. TREM2 triggers microglial density and age-related neuronal loss. Glia 67, 539–550 (2019).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017).
Stevens, B. et al. The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178 (2007).
Stephan, A. H. et al. A dramatic increase of C1q protein in the CNS during normal aging. J. Neurosci. 33, 13460–13474 (2013).
Hennessy, E., Griffin, É. W. & Cunningham, C. Astrocytes are primed by chronic neurodegeneration to produce exaggerated chemokine and cell infiltration responses to acute stimulation with the cytokines IL-1β and TNF-α. J. Neurosci. 35, 8411–8422 (2015).
Clarke, L. E. et al. Normal aging induces A1-like astrocyte reactivity. Proc. Natl Acad. Sci. USA 115, E1896–E1905 (2018).
Rappold, T. et al. Evidence of an association between brain cellular injury and cognitive decline after non-cardiac surgery. Br. J. Anaesth. 116, 83–89 (2016).
Lopez, M. G. et al. Intraoperative oxidative damage and delirium after cardiac surgery. Anesthesiology 132, 551–561 (2020).
Hall, R. J. et al. Delirium and cerebrospinal fluid S100B in hip fracture patients: a preliminary study. Am. J. Geriatr. Psychiatry 21, 1239–1243 (2013).
Hov, K. R. et al. Cerebrospinal fluid S100B and Alzheimer’s disease biomarkers in hip fracture patients with delirium. Dement. Geriatr. Cogn. Dis. Extra 7, 374–385 (2017).
Haruwaka, K. et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat. Commun. 10, 5816 (2019).
Serhan, C. N. & Levy, B. D. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J. Clin. Invest. 128, 2657–2669 (2018).
Serhan, C. N. & Savill, J. Resolution of inflammation: the beginning programs the end. Nat. Immunol. 6, 1191–1197 (2005).
Bannenberg, G. L. et al. Molecular circuits of resolution: formation and actions of resolvins and protectins. J. Immunol. 174, 4345–4355 (2005).
Malawista, S. E., de Boisfleury Chevance, A., van Damme, J. & Serhan, C. N. Tonic inhibition of chemotaxis in human plasma. Proc. Natl Acad. Sci. USA 105, 17949–17954 (2008).
Chiang, N. et al. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484, 524–528 (2012).
Terrando, N. et al. Aspirin-triggered resolvin D1 prevents surgery-induced cognitive decline. FASEB J. 27, 3564–3571 (2013).
Kooij, G. et al. Specialized pro-resolving lipid mediators are differentially altered in peripheral blood of patients with multiple sclerosis and attenuate monocyte and blood-brain barrier dysfunction. Haematologica 105, 2056–2070 (2020).
Marcos-Contreras, O. A. et al. Selective targeting of nanomedicine to inflamed cerebral vasculature to enhance the blood–brain barrier. Proc. Natl Acad. Sci. USA 117, 3405–3414 (2020).
Yousef, H. et al. Aged blood impairs hippocampal neural precursor activity and activates microglia via brain endothelial cell VCAM1. Nat. Med. 25, 988–1000 (2019).
Miller-Rhodes, P. et al. The broad spectrum mixed-lineage kinase 3 inhibitor URMC-099 prevents acute microgliosis and cognitive decline in a mouse model of perioperative neurocognitive disorders. J. Neuroinflammation 16, 193 (2019).
Ryu, J. K. et al. Fibrin-targeting immunotherapy protects against neuroinflammation and neurodegeneration. Nat. Immunol. 19, 1212–1223 (2018).
Acknowledgements
We thank Kathy Gage (Duke University Medical Center) for editorial assistance. T.Y. acknowledges a Department of Medicine Chair Research Award (Duke University) and an ASN Kidney Cure Carl W. Gottschalk Research Scholar Grant. N.T. is supported by NIA grants R01AG057525, R21 AG055877-01A1 and R03 AG064260-01; the Department of Anesthesiology; and Duke Institute of Brain Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information L. A. Dempsey was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, T., Velagapudi, R. & Terrando, N. Neuroinflammation after surgery: from mechanisms to therapeutic targets. Nat Immunol 21, 1319–1326 (2020). https://doi.org/10.1038/s41590-020-00812-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41590-020-00812-1
This article is cited by
-
Electrical stimulation of the vagus nerve improves amyloid pathology in delirium superimposed on dementia
Bioelectronic Medicine (2026)
-
Subanesthetic S-ketamine prevents postoperative delirium and reduces inflammatory cytokines in older patients receiving hip fracture surgery: a randomized, controlled study
Naunyn-Schmiedeberg's Archives of Pharmacology (2026)
-
Efficacy and safety of gastrodin in preventing postoperative delirium following cardiac surgery: a randomized placebo controlled clinical trial
Critical Care (2025)
-
Nanozymes in neuropathic pain: strategies bridging oxidative stress, mitochondrial repair, and neuroimmune modulation for targeted therapy
Journal of Neuroinflammation (2025)
-
Comparative effectiveness of monotherapy vs. combination therapy for postoperative central nervous system infections in neurosurgical patients: a retrospective cohort study
BMC Infectious Diseases (2025)