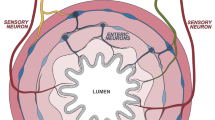
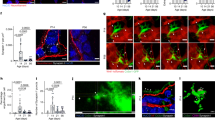
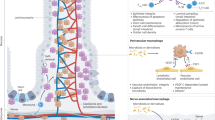
Abstract
Neuron–macrophage cross-talk in the intestine plays a crucial role in the maintenance of tissue homeostasis and the modulation of immune responses throughout life. Here, we describe how gut neuron–macrophage interactions shift macrophage phenotype and function from early development to adulthood and how this cross-talk modulates the macrophage function in response to infection and inflammation. We highlight how a neural microenvironment instructs a neuron-associated macrophage phenotype in the gut and show that their phenotype may resemble nerve-associated macrophages in other organs. Finally, we note that the loss of neuron-associated macrophages or a shift in their phenotype can contribute to enteric neurodegeneration in the gastrointestinal tract, causing gut motility disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Guilliams, M., Thierry, G. R., Bonnardel, J. & Bajenoff, M. Establishment and maintenance of the macrophage niche. Immunity 52, 434–451 (2020).
Sehgal, A., Irvine, K. M. & Hume, D. A. Functions of macrophage colony-stimulating factor (CSF1) in development, homeostasis, and tissue repair. Semin. Immunol. 54, 101509 (2021).
Wang, Y. et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immunol. 13, 753–760 (2012).
Gschwend, J. et al. Alveolar macrophages rely on GM-CSF from alveolar epithelial type 2 cells before and after birth. J. Exp. Med. 218, e20210745 (2021).
Bonnardel, J. et al. Stellate cells, hepatocytes, and endothelial cells imprint the kupffer cell identity on monocytes colonizing the liver macrophage niche. Immunity 51, 638–654 (2019).
De Schepper, S. et al. Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell 175, 400–415 (2018).
Honda, M. et al. Perivascular localization of macrophages in the intestinal mucosa is regulated by Nr4a1 and the microbiome. Nat. Commun. 11, 1329 (2020).
Muller, P. A. et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 158, 300–313 (2014).
Viola, M. F. et al. Dedicated macrophages organize and maintain the enteric nervous system. Nature 618, 818–826 (2023).
T’Jonck, W., Guilliams, M. & Bonnardel, J. Niche signals and transcription factors involved in tissue-resident macrophage development. Cell Immunol. 330, 43–53 (2018).
Varol, C., Mildner, A. & Jung, S. Macrophages: development and tissue specialization. Annu. Rev. Immunol. 33, 643–675 (2015).
Shaw, T. N. et al. Tissue-resident macrophages in the intestine are long lived and defined by Tim-4 and CD4 expression. J. Exp. Med. 215, 1507–1518 (2018).
Michel, K. et al. How big is the little brain in the gut? Neuronal numbers in the enteric nervous system of mice, Guinea pig, and human. Neurogastroenterol. Motil. 34, e14440 (2022).
Fung, C. & Vanden Berghe, P. Functional circuits and signal processing in the enteric nervous system. Cell. Mol. Life Sci. 77, 4505–4522 (2020).
Bronner, M. E. & LeDouarin, N. M. Development and evolution of the neural crest: an overview. Dev. Biol. 366, 2–9 (2012).
Cui, J., Michaille, J. J., Jiang, W. & Zile, M. H. Retinoid receptors and vitamin A deficiency: differential patterns of transcription during early avian development and the rapid induction of RARs by retinoic acid. Dev. Biol. 260, 496–511 (2003).
Simkin, J. E., Zhang, D., Rollo, B. N. & Newgreen, D. F. Retinoic acid upregulates ret and induces chain migration and population expansion in vagal neural crest cells to colonise the embryonic gut. PLoS ONE 8, e64077 (2013).
Fu, M., Lui, V. C. H., Sham, M. H., Cheung, A. N. Y. & Tam, P. K. H. HOXB5 expression is spatially and temporarily regulated in human embryonic gut during neural crest cell colonization and differentiation of enteric neuroblasts. Dev. Dyn. 228, 1–10 (2003).
Anderson, R. B., Stewart, A. L. & Young, H. M. Phenotypes of neural-crest-derived cells in vagal and sacral pathways. Cell Tissue Res. 323, 11–25 (2006).
Burns, A. J. & Le Douarin, N. M. The sacral neural crest contributes neurons and glia to the post-umbilical gut: spatiotemporal analysis of the development of the enteric nervous system. Development 125, 4335–4347 (1998).
Uesaka, T., Nagashimada, M. & Enomoto, H. Neuronal differentiation in schwann cell lineage underlies postnatal neurogenesis in the enteric nervous system. J. Neurosci. 35, 9879–9888 (2015).
Bergner, A. J. et al. Birthdating of myenteric neuron subtypes in the small intestine of the mouse. J. Comp. Neurol. 522, 514–527 (2014).
Memic, F. et al. Transcription and signaling regulators in developing neuronal subtypes of mouse and human enteric nervous system. Gastroenterology 154, 624–636 (2018).
Hao, M. M. & Young, H. M. Development of enteric neuron diversity. J. Cell. Mol. Med. 13, 1193–1210 (2009).
Nagy, N. & Goldstein, A. M. Enteric nervous system development: a crest cell’s journey from neural tube to colon. Semin. Cell Dev. Biol. 66, 94–106 (2017).
Parathan, P., Wang, Y., Leembruggen, A. J., Bornstein, J. C. & Foong, J. P. The enteric nervous system undergoes significant chemical and synaptic maturation during adolescence in mice. Dev. Biol. 458, 75–87 (2020).
Vries, Pde, Soret, R., Suply, E., Heloury, Y. & Neunlist, M. Postnatal development of myenteric neurochemical phenotype and impact on neuromuscular transmission in the rat colon. Am. J. Physiol. Gastrointest. Liver Physiol. 299, 539–547 (2010).
Avetisyan, M. et al. Muscularis macrophage development in the absence of an enteric nervous system. Proc. Natl Acad. Sci. USA 115, 4696–4701 (2018).
Dziabis, J. E. & Bilbo, S. D. Microglia and sensitive periods in brain development. Curr. Top. Behav. Neurosci. 53, 55–78 (2022).
Pendse, M. et al. Macrophages regulate gastrointestinal motility through complement component 1q. eLife 12, e78558 (2023).
Stevens, B. et al. The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178 (2007).
Kiss, M. et al. Junctional adhesion molecule-A is dispensable for myeloid cell recruitment and diversification in the tumor microenvironment. Front. Immunol. 13, 1003975 (2022).
Ydens, E. et al. Profiling peripheral nerve macrophages reveals two macrophage subsets with distinct localization, transcriptome and response to injury. Nat. Neurosci. 23, 676–689 (2020).
Wang, P. L. et al. Peripheral nerve resident macrophages share tissue-specific programming and features of activated microglia. Nat. Commun. 11, 2552 (2020).
Kolter, J., Kierdorf, K. & Henneke, P. Origin and differentiation of nerve-associated macrophages. J. Immunol. 204, 271–279 (2020).
Chakarov, S. et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science 363, eaau0964 (2019).
Pirzgalska, R. M. et al. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat. Med. 23, 1309–1318 (2017).
English, K. et al. The liver contains distinct interconnected networks of CX3CR1+ macrophages, XCR1+ type 1 and CD301a+ type 2 conventional dendritic cells embedded within portal tracts. Immunol. Cell Biol. 100, 394–408 (2022).
Ural, B. B. et al. Identification of a nerve-associated, lung-resident interstitial macrophage subset with distinct localization and immunoregulatory properties. Sci. Immunol. 5, eaax8756 (2020).
Butovsky, O. et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat. Neurosci. 17, 131–143 (2014).
Gosselin, D. et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 159, 1327–1340 (2014).
Bohlen, C. J. et al. Diverse requirements for microglial survival, specification, and function revealed by defined-medium cultures. Neuron 94, 759–773 (2017).
Parkhurst, C. N. et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155, 1596–1609 (2013).
Wlodarczyk, A. et al. A novel microglial subset plays a key role in myelinogenesis in developing brain. EMBO J. 36, 3292–3308 (2017).
Gabanyi, I. et al. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell 164, 378–391 (2016).
Wrana, J. L., Attisano, L., Wieser, R., Ventura, F. & Massagué, J. Mechanism of activation of the TGF-beta receptor. Nature 370, 341–347 (1994).
Ten Dijke, P., Miyazono, K. & Heldin, C. H. Signaling via hetero-oligomeric complexes of type I and type II serine/threonine kinase receptors. Curr. Opin. Cell Biol. 8, 139–145 (1996).
Massagué, J. & Wotton, D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 19, 1745–1754 (2000).
Arnold, T. D. et al. Impaired αVβ8 and TGFβ signaling lead to microglial dysmaturation and neuromotor dysfunction. J. Exp. Med. 216, 900–915 (2019).
Zöller, T. et al. Silencing of TGFβ signalling in microglia results in impaired homeostasis. Nat. Commun. 9, 4011 (2018).
Elmore, M. R. P. et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82, 380–397 (2014).
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010).
Dai, X.-M. et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood 99, 111–120 (2002).
Muffat, J. et al. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat. Med. 22, 1358–1367 (2016).
Blevins, G. & Fedoroff, S. Microglia in colony-stimulating factor 1-deficient op/op mice. J. Neurosci. Res. 40, 535–544 (1995).
Kondo, Y. & Duncan, I. D. Selective reduction in microglia density and function in the white matter of colony-stimulating factor-1-deficient mice. J. Neurosci. Res. 87, 2686–2695 (2009).
Greter, M. et al. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity 37, 1050–1060 (2012).
MacDonald, K. P. A. et al. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood 116, 3955–3963 (2010).
Tushinski, R. J. et al. Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell 28, 71–81 (1982).
Grubišić, V. et al. Enteric glia modulate macrophage phenotype and visceral sensitivity following inflammation. Cell Rep. 32, 108100 (2020).
Chalazonitis, A. et al. Bone morphogenetic protein regulation of enteric neuronal phenotypic diversity: relationship to timing of cell cycle exit. J. Comp. Neurol. 509, 474–492 (2008).
Stakenborg, N., Gomez-Pinilla, P. J. & Boeckxstaens, G. E. Postoperative ileus: pathophysiology, current therapeutic approaches. Handb. Exp. Pharmacol. 239, 39–57 (2017).
Türler, A. et al. Leukocyte-derived inducible nitric oxide synthase mediates murine postoperative ileus. Ann. Surg. 244, 220–229 (2006).
Kalff, J. C., Schraut, W. H., Billiar, T. R., Simmons, R. L. & Bauer, A. J. Role of inducible nitric oxide synthase in postoperative intestinal smooth muscle dysfunction in rodents. Gastroenterology 118, 316–327 (2000).
Schwarz, N. T. et al. Prostanoid production via COX-2 as a causative mechanism of rodent postoperative ileus. Gastroenterology 121, 1354–1371 (2001).
Wehner, S. et al. Inhibition of macrophage function prevents intestinal inflammation and postoperative ileus in rodents. Gut 56, 176–185 (2007).
Schneider, R. et al. A novel P2X2-dependent purinergic mechanism of enteric gliosis in intestinal inflammation. EMBO Mol. Med. 13, e12724 (2021).
Leven, P. et al. β-adrenergic signaling triggers enteric glial reactivity and acute enteric gliosis during surgery. J. Neuroinflammation 20, 255 (2023).
Rosenbaum, C. et al. Activation of myenteric glia during acute inflammation in vitro and in vivo. PLoS ONE 11, e0151335 (2016).
Liñán-Rico, A. et al. Molecular signaling and dysfunction of the human reactive enteric glial cell phenotype: implications for GI infection, IBD, POI, neurological, motility, and GI disorders. Inflamm. Bowel Dis. 22, 1812–1834 (2016).
Schneider, R. et al. IL-1-dependent enteric gliosis guides intestinal inflammation and dysmotility and modulates macrophage function. Commun. Biol. 5, 811 (2022).
Hupa, K. J. et al. AIM2 inflammasome-derived IL-1β induces postoperative ileus in mice. Sci. Rep. 9, 10602 (2019).
Stoffels, B. et al. Postoperative ileus involves interleukin-1 receptor signaling in enteric glia. Gastroenterology 146, 176–187 (2014).
Stakenborg, M. et al. Enteric glial cells favor accumulation of anti-inflammatory macrophages during the resolution of muscularis inflammation. Mucosal Immunol. 15, 1296–1308 (2022).
Chandrasekharan, B. & Srinivasan, S. Diabetes and the enteric nervous system. Neurogastroenterol. Motil. 19, 951–960 (2007).
Marathe, C. S., Rayner, C. K., Wu, T., Jones, K. L. & Horowitz, M. Gastrointestinal disorders in diabetes. In Endotext (eds. Feingold, K. R. et al.) (MDText.com, 2024).
Cipriani, G. et al. Diabetic Csf1op/op mice lacking macrophages are protected against the development of delayed gastric emptying. Cell. Mol. Gastroenterol. Hepatol. 11, 40–47 (2016).
Cipriani, G. et al. Change in populations of macrophages promotes development of delayed gastric emptying in mice. Gastroenterology 154, 2122–2136 (2018).
Choi, K. M. et al. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology 135, 2055–2064 (2008).
Choi, K. M. et al. CD206-positive M2 macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice. Gastroenterology 138, 2399–2409 (2010).
Bernard, C. E. et al. Association of low numbers of CD206-positive cells with loss of ICC in the gastric body of patients with diabetic gastroparesis. Neurogastroenterol. Motil. 26, 1275–1284 (2014).
Grover, M. et al. Diabetic and idiopathic gastroparesis is associated with loss of CD206-positive macrophages in the gastric antrum. Neurogastroenterol. Motil 29, e13018 (2017).
Chikkamenahalli, L. L. et al. Single cell atlas of human gastric muscle immune cells and macrophage-driven changes in idiopathic gastroparesis. iScience 27, 108991 (2024).
Grover, M. et al. Proteomics in gastroparesis: unique and overlapping protein signatures in diabetic and idiopathic gastroparesis. Am. J. Physiol. Gastrointest. Liver Physiol. 317, G716–G726 (2019).
Grover, M. et al. Transcriptomic signatures reveal immune dysregulation in human diabetic and idiopathic gastroparesis. BMC Med. Genomics 11, 62 (2018).
Choi, K. M. et al. Induction of heme oxygenase reverses diabetic gastroparesis in NOD/Ltj mice. Gastroenterology 134, A-123 (2008).
Geboes, K. & Collins, S. Structural abnormalities of the nervous system in Crohn’s disease and ulcerative colitis. Neurogastroenterol. Motil. 10, 189–202 (1998).
Sharkey, K. A. & Kroese, A. B. A. Consequences of intestinal inflammation on the enteric nervous system: neuronal activation induced by inflammatory mediators. Anat. Rec. 262, 79–90 (2001).
Stavely, R., Abalo, R. & Nurgali, K. Targeting enteric neurons and plexitis for the management of inflammatory bowel disease. Curr. Drug Targets 21, 1428–1439 (2020).
Lomax, A. E., Fernández, E. & Sharkey, K. A. Plasticity of the enteric nervous system during intestinal inflammation. Neurogastroenterol. Motil. 17, 4–15 (2005).
Spear, E. T. & Mawe, G. M. Enteric neuroplasticity and dysmotility in inflammatory disease: key players and possible therapeutic targets. Am. J. Physiol. Gastrointest. Liver Physiol. 317, G853–G861 (2019).
Galeazzi, F., Haapala, E. M., Van Rooijen, N., Vallance, B. A. & Collins, S. M. Inflammation-induced impairment of enteric nerve function in nematode-infected mice is macrophage dependent. Am. J. Physiol. Gastrointest. Liver Physiol. 278, G259–G265 (2000).
Jacobson, K., McHugh, K. & Collins, S. M. Experimental colitis alters myenteric nerve function at inflamed and noninflamed sites in the rat. Gastroenterology 109, 718–722 (1995).
Dora, D. et al. Evidence of a myenteric plexus barrier and its macrophage-dependent degradation during murine colitis: implications in enteric neuroinflammation. Cell Mol. Gastroenterol. Hepatol. 12, 1617–1641 (2021).
Ferrante, M. et al. The value of myenteric plexitis to predict early postoperative Crohn’s disease recurrence. Gastroenterology 130, 1595–1606 (2006).
Becker, L. et al. Age-dependent shift in macrophage polarisation causes inflammation-mediated degeneration of enteric nervous system. Gut 67, 827–836 (2017).
Bishop, E. S. et al. Age-dependent microglial disease phenotype results in functional decline in gut macrophages. Gastro. Hep. Adv. 2, 261–276 (2023).
Lavin, Y., Mortha, A., Rahman, A. & Merad, M. Regulation of macrophage development and function in peripheral tissues. Nat. Rev. Immunol. 15, 731–744 (2015).
Hammond, T. R. et al. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity 50, 253–271 (2019).
Norden, D. M. & Godbout, J. P. Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol. Appl. Neurobiol. 39, 19–34 (2013).
Cribbs, D. H. et al. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J. Neuroinflammation 9, 179 (2012).
Henry, C. J., Huang, Y., Wynne, A. M. & Godbout, J. P. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav. Immun. 23, 309–317 (2009).
Godbout, J. P. et al. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 19, 1329–1331 (2005).
Frank, M. G., Barrientos, R. M., Watkins, L. R. & Maier, S. F. Aging sensitizes rapidly isolated hippocampal microglia to LPS ex vivo. J. Neuroimmunol. 226, 181–184 (2010).
Moore, B. A. et al. Altered inflammatory gene expression underlies increased susceptibility to murine postoperative ileus with advancing age. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G1650–G1659 (2007).
Verheijden, S., Schepper, S., De & Boeckxstaens, G. E. Neuron-macrophage crosstalk in the intestine: a “microglia” perspective. Front. Cell Neurosci. 9, 1–6 (2015).
Matheis, F. et al. Adrenergic signaling in muscularis macrophages limits infection-induced neuronal loss. Cell 180, 64–78(2020).
Stakenborg, N., Giovangiulio, M., Di, Boeckxstaens, G. E. & Matteoli, G. The versatile role of the vagus nerve in the gastrointestinal tract. EMJ Gastroenterol. Gastroenterol. 2013 1, 106–114 (2013).
Stakenborg, N., Viola, M. F. & Boeckxstaens, G. E. Intestinal neuro-immune interactions: focus on macrophages, mast cells and innate lymphoid cells. Curr. Opin. Neurobiol. 62, 68–75 (2020).
Stakenborg, N. & Boeckxstaens, G. E. Bioelectronics in the brain–gut axis: focus on inflammatory bowel disease (IBD). Int. Immunol. 33, 337–348 (2021).
Cailotto, C. et al. Neuro-anatomical evidence indicating indirect modulation of macrophages by vagal efferents in the intestine but not in the spleen. PLoS ONE 9, e87785 (2014).
Goehler, L. E. et al. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav. Immun. 19, 334–344 (2005).
Matteoli, G. et al. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut 63, 938–948 (2013).
de Jonge, W. J. et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2–STAT3 signaling pathway. Nat. Immunol. 6, 844–851 (2005).
Stakenborg, N. et al. Abdominal vagus nerve stimulation as a new therapeutic approach to prevent postoperative ileus. Neurogastroenterol. Motil. https://doi.org/10.1111/nmo.13075 (2017).
Stakenborg, N. et al. Preoperative administration of the 5-HT4 receptor agonist prucalopride reduces intestinal inflammation and shortens postoperative ileus via cholinergic enteric neurons. Gut 68, 1406–1416 (2019).
Meroni, E. et al. Vagus nerve stimulation promotes epithelial proliferation and controls colon monocyte infiltration during DSS-induced colitis. Front. Med. 8, 694268 (2021).
Bonaz, B. et al. Chronic vagus nerve stimulation in Crohn’s disease: a 6-month follow-up pilot study. Neurogastroenterol. Motil. 28, 948–953 (2016).
Meroni, E. et al. Functional characterization of oxazolone-induced colitis and survival improvement by vagus nerve stimulation. PLoS ONE 13, e0197487 (2018).
Sinniger, V. et al. A 12-month pilot study outcomes of vagus nerve stimulation in Crohn’s disease. Neurogastroenterol. Motil. 32, e13911 (2020).
D’Haens, G. et al. The effects of vagus nerve stimulation in biologic-refractory Crohn’s disease: a prospective clinical trial. J. Crohns Colitis 12, S397–S398 (2018).
Payne, S. C. et al. Anti-inflammatory effects of abdominal vagus nerve stimulation on experimental intestinal inflammation. Front. Neurosci. 13, 418 (2019).
Caravaca, A. S. et al. An effective method for acute vagus nerve stimulation in experimental inflammation. Front. Neurosci. 13, 877 (2019).
Di Giovangiulio, M. et al. Vagotomy affects the development of oral tolerance and increases susceptibility to develop colitis independently of the alpha-7 nicotinic receptor. Mol. Med. 22, 464–476 (2016).
Domanska, D. et al. Single-cell transcriptomic analysis of human colonic macrophages reveals niche-specific subsets. J. Exp. Med. 219, e20211846 (2022).
Bujko, A. et al. Transcriptional and functional profiling defines human small intestinal macrophage subsets. J. Exp. Med. 215, 441–458 (2018).
Bain, C. C. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 6, 498–510 (2013).
Acknowledgements
This work was supported by the European Research Council (ERC) Advanced Grant (ERC- 833816-NEUMACS) to G.B. M.F.V is supported by an EMBO postdoctoral fellowship (ALTF 873-2023).
Author information
Authors and Affiliations
Contributions
N.S., M.F.V. and G.B. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks John Grainger and Gerard Eberl for their contribution to the peer review of this work. Primary Handling Editor: S. Houston in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stakenborg, N., Viola, M.F. & Boeckxstaens, G. Intestinal neuron-associated macrophages in health and disease. Nat Immunol 26, 1004–1013 (2025). https://doi.org/10.1038/s41590-025-02150-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41590-025-02150-6