Abstract
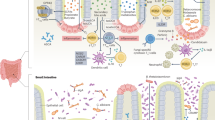
Inflammatory bowel disease (IBD) is a complex, multifactorial inflammatory disorder of the gut characterized by an imbalance in host–microbiota interactions. Here, we review how early events of IBD are shaped by gene–environment interactions, especially those involving microbial perturbations. Those perturbations eventually lead to chronic inflammation and tissue damage of the gastrointestinal tract. IBD is a multi-hit process in which infectious and noninfectious agents initiate a cascade of immune activation in genetically susceptible individuals. Ultimately the process results in irreversible immunological and physical scarring. These interactions are host specific, with genetic variants influencing the threshold for immune activation and the degree of damage, thus leading to variability in disease progression and therapeutic outcomes. Finally, we discuss challenges, including addressing health disparities and potential strategies for more personalized and effective therapies that target host–microbiota interactions during the preclinical phase of IBD.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Rudbaek, J. J. et al. Deciphering the different phases of preclinical inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 21, 86–100 (2023).
Ramanan, D. & Cadwell, K. Intrinsic defense mechanisms of the intestinal epithelium. Cell Host Microbe 19, 434–441 (2016).
Chikina, A. & Matic Vignjevic, D. At the right time in the right place: how do luminal gradients position the microbiota along the gut? Cells Dev. 168, 203712 (2021).
Gu, Y. et al. Immune microniches shape intestinal Treg function. Nature 628, 854–862 (2024).
Hoytema van Konijnenburg, D. P. et al. Intestinal epithelial and intraepithelial T cell crosstalk mediates a dynamic response to infection. Cell 171, 783–794 (2017).
Edelblum, K. L. et al. γδ intraepithelial lymphocyte migration limits transepithelial pathogen invasion and systemic disease in mice. Gastroenterology 148, 1417–1426 (2015).
Koch, M. A. et al. Maternal IgG and IgA antibodies dampen mucosal T helper cell responses in early life. Cell 165, 827–841 (2016).
Bunker, J. J. et al. Innate and adaptive humoral responses coat distinct commensal bacteria with immunoglobulin A. Immunity 43, 541–553 (2015).
Vujkovic-Cvijin, I. et al. The systemic anti-microbiota IgG repertoire can identify gut bacteria that translocate across gut barrier surfaces. Sci. Transl. Med. 14, eabl3927 (2022).
Palm, N. W. et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 158, 1000–1010 (2014).
Nagashima, K. et al. Mapping the T cell repertoire to a complex gut bacterial community. Nature 621, 162–170 (2023).
Sefik, E. et al. Mucosal immunology. Individual intestinal symbionts induce a distinct population of RORγ+ regulatory T cells. Science 349, 993–997 (2015).
Atarashi, K. et al. TH17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell 163, 367–380 (2015).
Sano, T. et al. An IL-23R/IL-22 circuit regulates epithelial serum amyloid A to promote local effector TH17 responses. Cell 164, 324 (2016).
Xu, M. et al. c-MAF-dependent regulatory T cells mediate immunological tolerance to a gut pathobiont. Nature 554, 373–377 (2018).
Chai, J. N. et al. Helicobacter species are potent drivers of colonic T cell responses in homeostasis and inflammation. Sci. Immunol. 2, eaal5068 (2017).
Brodin, P. & Davis, M. M. Human immune system variation. Nat. Rev. Immunol. 17, 21–29 (2017).
Bakker, O. B. et al. Integration of multi-omics data and deep phenotyping enables prediction of cytokine responses. Nat. Immunol. 19, 776–786 (2018).
Brodin, P. et al. Variation in the human immune system is largely driven by non-heritable influences. Cell 160, 37–47 (2015).
Lin, J. D. et al. Rewilding Nod2 and Atg16l1 mutant mice uncovers genetic and environmental contributions to microbial responses and immune cell composition. Cell Host Microbe 27, 830–840 (2020).
Yeung, F. et al. Altered immunity of laboratory mice in the natural environment is associated with fungal colonization. Cell Host Microbe 27, 809–822 (2020).
Carr, E. J. et al. The cellular composition of the human immune system is shaped by age and cohabitation. Nat. Immunol. 17, 461–468 (2016).
Patin, E. et al. Natural variation in the parameters of innate immune cells is preferentially driven by genetic factors. Nat. Immunol. 19, 302–314 (2018).
Saint-Andre, V. et al. Smoking changes adaptive immunity with persistent effects. Nature 626, 827–835 (2024).
Cader, M. Z. et al. FAMIN is a multifunctional purine enzyme enabling the purine nucleotide cycle. Cell 180, 278–295 (2020).
Oyesola, O. et al. Genetic and environmental interactions contribute to immune variation in rewilded mice. Nat. Immunol. 25, 1270–1282 (2024).
Zheng, H. B., de la Morena, M. T. & Suskind, D. L. The growing need to understand very early onset inflammatory bowel disease. Front Immunol. 12, 675186 (2021).
Bolton, C. et al. An integrated taxonomy for monogenic inflammatory bowel disease. Gastroenterology 162, 859–876 (2022).
Sazonovs, A. et al. Large-scale sequencing identifies multiple genes and rare variants associated with Crohn’s disease susceptibility. Nat. Genet. 54, 1275–1283 (2022).
Stankey, C. T. et al. A disease-associated gene desert directs macrophage inflammation through ETS2. Nature 630, 447–456 (2024).
Keestra-Gounder, A. M. et al. NOD1 and NOD2 signalling links ER stress with inflammation. Nature 532, 394–397 (2016).
Lupfer, C. et al. Receptor interacting protein kinase 2-mediated mitophagy regulates inflammasome activation during virus infection. Nat. Immunol. 14, 480–488 (2013).
Petnicki-Ocwieja, T. et al. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc. Natl Acad. Sci. USA 106, 15813–15818 (2009).
Couturier-Maillard, A. et al. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J. Clin. Invest. 123, 700–711 (2013).
Caruso, R. et al. A specific gene–microbe interaction drives the development of Crohn’s disease-like colitis in mice. Sci. Immunol. 4, eaaw4341 (2019).
Goethel, A. et al. Nod2 influences microbial resilience and susceptibility to colitis following antibiotic exposure. Mucosal Immunol. 12, 720–732 (2019).
Ramanan, D., Tang, M. S., Bowcutt, R., Loke, P. & Cadwell, K. Bacterial sensor Nod2 prevents inflammation of the small intestine by restricting the expansion of the commensal Bacteroides vulgatus. Immunity 41, 311–324 (2014).
Nayar, S. et al. A myeloid–stromal niche and gp130 rescue in NOD2-driven Crohn’s disease. Nature 593, 275–281 (2021).
Kim, Y. G. et al. The Nod2 sensor promotes intestinal pathogen eradication via the chemokine CCL2-dependent recruitment of inflammatory monocytes. Immunity 34, 769–780 (2011).
Ramanan, D. et al. Helminth infection promotes colonization resistance via type 2 immunity. Science 352, 608–612 (2016).
Hugot, J. P. et al. Prevalence of CARD15/NOD2 mutations in Caucasian healthy people. Am. J. Gastroenterol. 102, 1259–1267 (2007).
Jang, K. K. et al. Antimicrobial overproduction sustains intestinal inflammation by inhibiting Enterococcus colonization. Cell Host Microbe 31, 1450–1468 (2023).
Zhou, L. et al. Innate lymphoid cells support regulatory T cells in the intestine through interleukin-2. Nature 568, 405–409 (2019).
Gao, J. et al. Gut microbial DL-endopeptidase alleviates Crohn’s disease via the NOD2 pathway. Cell Host Microbe 30, 1435–1449 (2022).
Travassos, L. H. et al. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat. Immunol. 11, 55–62 (2010).
Cooney, R. et al. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat. Med. 16, 90–97 (2010).
Matsuzawa-Ishimoto, Y., Hwang, S. & Cadwell, K. Autophagy and inflammation. Annu. Rev. Immunol. 36, 73–101 (2018).
Chu, H. et al. Gene–microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science 352, 1116–1120 (2016).
Marchiando, A. M. et al. A deficiency in the autophagy gene Atg16L1 enhances resistance to enteric bacterial infection. Cell Host Microbe 14, 216–224 (2013).
Martin, P. K. et al. Autophagy proteins suppress protective type I interferon signalling in response to the murine gut microbiota. Nat. Microbiol. 3, 1131–1141 (2018).
Saitoh, T. et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1β production. Nature 456, 264–268 (2008).
Lahiri, A., Hedl, M. & Abraham, C. MTMR3 risk allele enhances innate receptor-induced signaling and cytokines by decreasing autophagy and increasing caspase-1 activation. Proc. Natl Acad. Sci. USA 112, 10461–10466 (2015).
Cadwell, K. et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456, 259–263 (2008).
Matsuzawa-Ishimoto, Y. et al. Autophagy protein ATG16L1 prevents necroptosis in the intestinal epithelium. J. Exp. Med. 214, 3687–3705 (2017).
Matsuzawa-Ishimoto, Y. et al. An intestinal organoid-based platform that recreates susceptibility to T cell-mediated tissue injury. Blood 135, 2388–2401 (2020).
Cadwell, K. et al. Virus-plus-susceptibility gene interaction determines Crohn’s disease gene Atg16L1 phenotypes in intestine. Cell 141, 1135–1145 (2010).
Bel, S. et al. Paneth cells secrete lysozyme via secretory autophagy during bacterial infection of the intestine. Science 357, 1047–1052 (2017).
Burger, E. et al. Loss of Paneth cell autophagy causes acute susceptibility to Toxoplasma gondii-mediated inflammation. Cell Host Microbe 23, 177–190 (2018).
Adolph, T. E. et al. Paneth cells as a site of origin for intestinal inflammation. Nature 503, 272–276 (2013).
Kaser, A. et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134, 743–756 (2008).
Liu, T. C. et al. Interaction between smoking and ATG16L1T300A triggers Paneth cell defects in Crohn’s disease. J. Clin. Invest. 128, 5110–5122 (2018).
Neil, J. A. et al. IFN-I and IL-22 mediate protective effects of intestinal viral infection. Nat. Microbiol. 4, 1737–1749 (2019).
Kernbauer, E., Ding, Y. & Cadwell, K. An enteric virus can replace the beneficial function of commensal bacteria. Nature 516, 94–98 (2014).
Matsuzawa-Ishimoto, Y. et al. The γδ IEL effector API5 masks genetic susceptibility to Paneth cell death. Nature 610, 547–554 (2022).
Xu, W. et al. Dysregulation of gammadelta intraepithelial lymphocytes precedes Crohn’s disease-like ileitis. Sci. Immunol. 10, eadk7429 (2025).
Dart, R. J. et al. Conserved gammadelta T cell selection by BTNL proteins limits progression of human inflammatory bowel disease. Science 381, eadh0301 (2023).
Jaeger, N. et al. Single-cell analyses of Crohn’s disease tissues reveal intestinal intraepithelial T cells heterogeneity and altered subset distributions. Nat. Commun. 12, 1921 (2021).
Sun, L. et al. Type I interferons link viral infection to enhanced epithelial turnover and repair. Cell Host Microbe 17, 85–97 (2014).
Mehto, S. et al. The Crohn’s disease risk factor IRGM limits NLRP3 inflammasome activation by impeding its assembly and by mediating its selective autophagy. Mol. Cell 73, 429–445 (2019).
Doron, I. et al. Human gut mycobiota tune immunity via CARD9-dependent induction of anti-fungal IgG antibodies. Cell 184, 1017–1031 (2021).
Lamas, B. et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 22, 598–605 (2016).
Zhang, Q. et al. Commensal bacteria direct selective cargo sorting to promote symbiosis. Nat. Immunol. 16, 918–926 (2015).
Sun, S. et al. Macrophage LRRK2 hyperactivity impairs autophagy and induces Paneth cell dysfunction. Sci. Immunol. 9, eadi7907 (2024).
Tasegian, A. et al. LRRK2 is not required for lysozyme expression in Paneth cells. Nat. Immunol. 25, 2037–2039 (2024).
Hedl, M., Zheng, S. & Abraham, C. The IL18RAP region disease polymorphism decreases IL-18RAP/IL-18R1/IL-1R1 expression and signaling through innate receptor-initiated pathways. J. Immunol. 192, 5924–5932 (2014).
Taylor, G. A. et al. Irgm1-deficiency leads to myeloid dysfunction in colon lamina propria and susceptibility to the intestinal pathogen Citrobacter rodentium. PLoS Pathog. 16, e1008553 (2020).
Jarret, A. et al. Enteric nervous system-derived IL-18 orchestrates mucosal barrier immunity. Cell 180, 50–63 (2020).
Shouval, D. S. et al. Interleukin 1beta mediates intestinal inflammation in mice and patients with interleukin 10 receptor deficiency. Gastroenterology 151, 1100–1104 (2016).
Shouval, D. S. et al. Enhanced TH17 responses in patients with IL10 receptor deficiency and infantile-onset IBD. Inflamm. Bowel Dis. 23, 1950–1961 (2017).
Mohanan, V. et al. C1orf106 is a colitis risk gene that regulates stability of epithelial adherens junctions. Science 359, 1161–1166 (2018).
Luong, P. et al. INAVA-ARNO complexes bridge mucosal barrier function with inflammatory signaling. eLife 7, e38539 (2018).
Rutgeerts, P. et al. Effect of faecal stream diversion on recurrence of Crohn’s disease in the neoterminal ileum. Lancet 338, 771–774 (1991).
Britton, G. J. et al. Microbiotas from humans with inflammatory bowel disease alter the balance of gut TH17 and RORγt+ regulatory T cells and exacerbate colitis in mice. Immunity 50, 212–224 (2019).
Ananthakrishnan, A. N., Whelan, K., Allegretti, J. R. & Sokol, H. Diet and microbiome-directed therapy 2.0 for IBD. Clin. Gastroenterol. Hepatol. 23, 406–418 (2024).
Peery, A. F. et al. AGA clinical practice guideline on fecal microbiota-based therapies for select gastrointestinal diseases. Gastroenterology 166, 409–434 (2024).
Aschard, H. et al. Genetic effects on the commensal microbiota in inflammatory bowel disease patients. PLoS Genet. 15, e1008018 (2019).
Kelly, C. J. et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17, 662–671 (2015).
Arpaia, N. et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 (2013).
Atarashi, K. et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500, 232–236 (2013).
Smith, P. M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013).
Byndloss, M. X. et al. Microbiota-activated PPAR-gamma signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 357, 570–575 (2017).
Atarashi, K. et al. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science 358, 359–365 (2017).
Rojas-Tapias, D. F. et al. Inflammation-associated nitrate facilitates ectopic colonization of oral bacterium Veillonella parvula in the intestine. Nat. Microbiol. 7, 1673–1685 (2022).
Kumbhari, A. et al. Discovery of disease-adapted bacterial lineages in inflammatory bowel diseases. Cell Host Microbe 32, 1147–1162 (2024).
Cao, Y. et al. Commensal microbiota from patients with inflammatory bowel disease produce genotoxic metabolites. Science 378, eabm3233 (2022).
Lopez, C. A. et al. Virulence factors enhance Citrobacter rodentium expansion through aerobic respiration. Science 353, 1249–1253 (2016).
Winter, S. E. et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 339, 708–711 (2013).
Ladinsky, M. S. et al. Endocytosis of commensal antigens by intestinal epithelial cells regulates mucosal T cell homeostasis. Science 363, eaat4042 (2019).
Lin, X. et al. IL-17RA-signaling in Lgr5+ intestinal stem cells induces expression of transcription factor ATOH1 to promote secretory cell lineage commitment. Immunity 55, 237–253 (2022).
Lee, J. S. et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity 43, 727–738 (2015).
Maxwell, J. R. et al. Differential roles for interleukin-23 and interleukin-17 in intestinal immunoregulation. Immunity 43, 739–750 (2015).
Song, X. et al. Growth factor FGF2 cooperates with interleukin-17 to repair intestinal epithelial damage. Immunity 43, 488–501 (2015).
Kumar, P. et al. Intestinal interleukin-17 receptor signaling mediates reciprocal control of the gut microbiota and autoimmune inflammation. Immunity 44, 659–671 (2016).
Hueber, W. et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 61, 1693–1700 (2012).
Diehl, G. E. et al. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX3CR1hi cells. Nature 494, 116–120 (2013).
Kim, M. et al. Critical role for the microbiota in CX3CR1+ intestinal mononuclear phagocyte regulation of intestinal T cell responses. Immunity 49, 151–163 (2018).
Ha, C. W. Y. et al. Translocation of viable gut microbiota to mesenteric adipose drives formation of creeping fat in humans. Cell 183, 666–683 (2020).
Wu, J. et al. Microbiota-induced alteration of kynurenine metabolism in macrophages drives formation of creeping fat in Crohn’s disease. Cell Host Microbe 32, 1927–1943 (2024).
Yu, S. et al. Paneth cell-derived lysozyme defines the composition of mucolytic microbiota and the inflammatory tone of the intestine. Immunity 53, 398–416 (2020).
Limon, J. J. et al. Malassezia is associated with Crohn’s disease and exacerbates colitis in mouse models. Cell Host Microbe 25, 377–388 (2019).
Jain, U. et al. Debaryomyces is enriched in Crohn’s disease intestinal tissue and impairs healing in mice. Science 371, 1154–1159 (2021).
Li, X. V. et al. Immune regulation by fungal strain diversity in inflammatory bowel disease. Nature 603, 672–678 (2022).
Ost, K. S. et al. Adaptive immunity induces mutualism between commensal eukaryotes. Nature 596, 114–118 (2021).
Savage, H. P. et al. Epithelial hypoxia maintains colonization resistance against Candida albicans. Cell Host Microbe 32, 1103–1113 (2024).
Howitt, M. R. et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 351, 1329–1333 (2016).
Gerrick, E. R. et al. Metabolic diversity in commensal protists regulates intestinal immunity and trans-kingdom competition. Cell 187, 62–78 (2024).
Chudnovskiy, A. et al. Host–protozoan interactions protect from mucosal infections through activation of the inflammasome. Cell 167, 444–456 (2016).
Dallari, S. et al. Enteric viruses evoke broad host immune responses resembling those elicited by the bacterial microbiome. Cell Host Microbe 29, 1014–1029 (2021).
Yang, J. Y. et al. Enteric viruses ameliorate gut inflammation via Toll-like receptor 3 and Toll-like receptor 7-mediated interferon-beta production. Immunity 44, 889–900 (2016).
Boland, B. S. et al. Heterogeneity and clonal relationships of adaptive immune cells in ulcerative colitis revealed by single-cell analyses. Sci. Immunol. 5, eabb4432 (2020).
Uzzan, M. et al. Ulcerative colitis is characterized by a plasmablast-skewed humoral response associated with disease activity. Nat. Med. 28, 766–779 (2022).
Hand, T. W. et al. Acute gastrointestinal infection induces long-lived microbiota-specific T cell responses. Science 337, 1553–1556 (2012).
Alexander, K. L. et al. Human microbiota flagellins drive adaptive immune responses in Crohn’s disease. Gastroenterology 161, 522–535 (2021).
Pedersen, T. K. et al. The CD4+ T cell response to a commensal-derived epitope transitions from a tolerant to an inflammatory state in Crohn’s disease. Immunity 55, 1909–1923 (2022).
Martini, G. R. et al. Selection of cross-reactive T cells by commensal and food-derived yeasts drives cytotoxic TH1 cell responses in Crohn’s disease. Nat. Med. 29, 2602–2614 (2023).
Fonseca, D. M. et al. Microbiota-dependent sequelae of acute infection compromise tissue-specific immunity. Cell 163, 354–366 (2015).
Czepielewski, R. S. et al. Ileitis-associated tertiary lymphoid organs arise at lymphatic valves and impede mesenteric lymph flow in response to tumor necrosis factor. Immunity 54, 2795–2811 (2021).
Carmody, R. N., Varady, K. & Turnbaugh, P. J. Digesting the complex metabolic effects of diet on the host and microbiome. Cell 187, 3857–3876 (2024).
Link, V. M. et al. Differential peripheral immune signatures elicited by vegan versus ketogenic diets in humans. Nat. Med. 30, 560–572 (2024).
Hashash, J. G., Elkins, J., Lewis, J. D. & Binion, D. G. AGA clinical practice update on diet and nutritional therapies in patients with inflammatory bowel disease: expert review. Gastroenterology 166, 521–532 (2024).
Desai, M. S. et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 167, 1339–1353 (2016).
Pereira, G. V. et al. Opposing diet, microbiome, and metabolite mechanisms regulate inflammatory bowel disease in a genetically susceptible host. Cell Host Microbe 32, 527–542 (2024).
Chassaing, B. et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 519, 92–96 (2015).
Wegorzewska, M. M. et al. Diet modulates colonic T cell responses by regulating the expression of a Bacteroides thetaiotaomicron antigen. Sci. Immunol. 4, eaau9079 (2019).
Lei, C. et al. Bacterial and host fucosylation maintain IgA homeostasis to limit intestinal inflammation in mice. Nat. Microbiol. 10, 126–143 (2025).
Rausch, P. et al. Colonic mucosa-associated microbiota is influenced by an interaction of Crohn disease and FUT2 (Secretor) genotype. Proc. Natl Acad. Sci. USA 108, 19030–19035 (2011).
Kamioka, M. et al. Intestinal commensal microbiota and cytokines regulate Fut2+ Paneth cells for gut defense. Proc. Natl Acad. Sci. USA 119, e2115230119 (2022).
Devkota, S. et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 487, 104–108 (2012).
Liu, T. C. et al. Western diet induces Paneth cell defects through microbiome alterations and farnesoid X receptor and type I interferon activation. Cell Host Microbe 29, 988–1001 (2021).
Hang, S. et al. Bile acid metabolites control TH17 and Treg cell differentiation. Nature 576, 143–148 (2019).
Paik, D. et al. Human gut bacteria produce TH17-modulating bile acid metabolites. Nature 603, 907–912 (2022).
Song, X. et al. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature 577, 410–415 (2020).
Lee, J. Y. et al. High-fat diet and antibiotics cooperatively impair mitochondrial bioenergetics to trigger dysbiosis that exacerbates pre-inflammatory bowel disease. Cell Host Microbe 28, 273–284 (2020).
Culp, E. J., Nelson, N. T., Verdegaal, A. A. & Goodman, A. L. Microbial transformation of dietary xenobiotics shapes gut microbiome composition. Cell 187, 6327–6345 (2024).
Sanmarco, L. M. et al. Identification of environmental factors that promote intestinal inflammation. Nature 611, 801–809 (2022).
Barnes, E. L., Loftus, E. V. Jr. & Kappelman, M. D. Effects of race and ethnicity on diagnosis and management of inflammatory bowel diseases. Gastroenterology 160, 677–689 (2021).
Khalessi, A. et al. Differential manifestations of inflammatory bowel disease based on race and immigration status. Gastro Hep. Adv. 3, 326–332 (2024).
Liu, Z. et al. Genetic architecture of the inflammatory bowel diseases across East Asian and European ancestries. Nat. Genet. 55, 796–806 (2023).
Gettler, K. et al. Common and rare variant prediction and penetrance of IBD in a large, multi-ethnic, health system-based biobank cohort. Gastroenterology 160, 1546–1557 (2021).
Astore, C. et al. The role of admixture in the rare variant contribution to inflammatory bowel disease. Genome Med. 15, 97 (2023).
Misra, R., Faiz, O., Munkholm, P., Burisch, J. & Arebi, N. Epidemiology of inflammatory bowel disease in racial and ethnic migrant groups. World J. Gastroenterol. 24, 424–437 (2018).
Damas, O. M. et al. Inflammatory bowel disease is presenting sooner after immigration in more recent US immigrants from Cuba. Aliment. Pharm. Ther. 46, 303–309 (2017).
Ip, W. K. E., Hoshi, N., Shouval, D. S., Snapper, S. & Medzhitov, R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 356, 513–519 (2017).
Torres, J. et al. Serum biomarkers identify patients who will develop inflammatory bowel diseases up to 5 years before diagnosis. Gastroenterology 159, 96–104 (2020).
Axelrad, J. E., Cadwell, K. H., Colombel, J. F. & Shah, S. C. Systematic review: gastrointestinal infection and incident inflammatory bowel disease. Aliment. Pharm. Ther. 51, 1222–1232 (2020).
Axelrad, J. E. et al. Gastrointestinal infection increases odds of inflammatory bowel disease in a nationwide case–control study. Clin. Gastroenterol. Hepatol. 17, 1311–1322 (2019).
Galipeau, H. J. et al. Novel fecal biomarkers that precede clinical diagnosis of ulcerative colitis. Gastroenterology 160, 1532–1545 (2021).
Nandy, A. et al. Epstein–Barr Virus (EBV) exposure precedes cCrohn’s disease development. Gastroenterology https://doi.org/10.1053/j.gastro.2025.01.247 (2025).
Lee, J. C. et al. Genome-wide association study identifies distinct genetic contributions to prognosis and susceptibility in Crohn’s disease. Nat. Genet. 49, 262–268 (2017).
Kugathasan, S. et al. Prediction of complicated disease course for children newly diagnosed with Crohn’s disease: a multicentre inception cohort study. Lancet 389, 1710–1718 (2017).
Schett, G., McInnes, I. B. & Neurath, M. F. Reframing immune-mediated inflammatory diseases through signature cytokine hubs. N. Engl. J. Med. 385, 628–639 (2021).
Canales-Herrerias, P. et al. Gut-associated lymphoid tissue attrition associates with response to anti-α4β7 therapy in ulcerative colitis. Sci. Immunol. 9, eadg7549 (2024).
Canale, V. et al. PTPN2 is a critical regulator of ileal paneth cell viability and function in mice. Cell Mol. Gastroenterol. Hepatol. 16, 39–62 (2023).
Bamias, G., Menghini, P., Pizarro, T. T. & Cominelli, F. Targeting TL1A and DR3: the new frontier of anti-cytokine therapy in IBD. Gut 74, 652–668 (2024).
Castellanos, J. G. et al. Microbiota-induced TNF-like ligand 1A drives group 3 innate lymphoid cell-mediated barrier protection and intestinal T cell activation during colitis. Immunity 49, 1077–1089 (2018).
Jacob, N. et al. Inflammation-independent TL1A-mediated intestinal fibrosis is dependent on the gut microbiome. Mucosal Immunol. 11, 1466–1476 (2018).
Jang, K. K. et al. Tofacitinib uptake by patient-derived intestinal organoids predicts individual clinical responsiveness. Gastroenterology 167, 1453–1456 (2024).
Khan, M. T. et al. Synergy and oxygen adaptation for development of next-generation probiotics. Nature 620, 381–385 (2023).
Broadhurst, M. J. et al. IL-22+ CD4+ T cells are associated with therapeutic Trichuris trichiura infection in an ulcerative colitis patient. Sci. Transl. Med. 2, 60ra88 (2010).
Maizels, R. M. Regulation of immunity and allergy by helminth parasites. Allergy 75, 524–534 (2020).
Federici, S. et al. Targeted suppression of human IBD-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation. Cell 185, 2879–2898 (2022).
Furuichi, M. et al. Commensal consortia decolonize Enterobacteriaceae via ecological control. Nature 633, 878–886 (2024).
Talbot, J. et al. Feeding-dependent VIP neuron-ILC3 circuit regulates the intestinal barrier. Nature 579, 575–580 (2020).
Friedrich, M. et al. IL-1-driven stromal-neutrophil interactions define a subset of patients with inflammatory bowel disease that does not respond to therapies. Nat. Med. 27, 1970–1981 (2021).
Acknowledgements
This work was supported by National Institutes of Health (NIH) grant DK093668 (to K.C.) and the Intramural Research Program of the National Institute of Allergy and Infectious Diseases at the NIH (to P.L.).
Author information
Authors and Affiliations
Contributions
K.C. and P.L. wrote and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
K.C. has received research support from Pfizer, Takeda, Genentech and AbbVie. K.C. has consulted for or received an honorarium from PureTech Health, Genentech and AbbVie. K.C. is an inventor on US patents 10,722,600 and provisional patent 62/935,035 and 63/157,225. P.L. declares no competing interests.
Peer review
Peer review information
Nature Immunology thanks the anonymous reviewers for their contribution to this work. Primary Handling Editor: P. Jauregui, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cadwell, K., Loke, P. Gene–environment interactions shape the host–microbial interface in inflammatory bowel disease. Nat Immunol 26, 1023–1035 (2025). https://doi.org/10.1038/s41590-025-02197-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41590-025-02197-5