Abstract
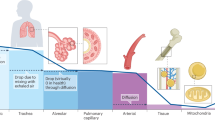
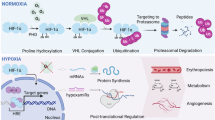
Oxygen availability and fluctuation are common changes in tissues and organs undergoing infection and damage. While acute hypoxia can rapidly alter immune cell metabolism and activity, chronic hypoxia can induce long-lasting changes in immune responses via oxygen-guided adaptation in signaling cascades and epitranscriptomic programs. These adaptations are orchestrated mainly by oxygen-sensing hydroxylases and oxygen-sensing epigenetic modifiers that regulate downstream hypoxia-inducible factor pathways and epigenetic reprogramming. In this Review, we summarize how acute and chronic hypoxia influence innate immune cell function and metabolism, thereby tailoring immune cell behavior within the tissue microenvironment. We further highlight the dual roles of hypoxia in regulating innate immune cell function in different (patho)physiological contexts and evaluate therapeutic strategies that target oxygen-sensing pathways to restore immune competence and tissue homeostasis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
McKeown, S. R. Defining normoxia, physoxia and hypoxia in tumours—implications for treatment response. Br. J. Radiol. 87, 20130676 (2014).
Zheng, L., Kelly, C. J. & Colgan, S. P. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A review in the theme: cellular responses to hypoxia. Am. J. Physiol. Cell Physiol. 309, C350–C360 (2015).
Spencer, J. A. et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 508, 269–273 (2014).
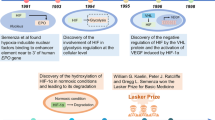
Schofield, C. J. & Ratcliffe, P. J. Oxygen sensing by HIF hydroxylases. Nat. Rev. Mol. Cell Biol. 5, 343–354 (2004).
Xiao, M. et al. Inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 26, 1326–1338 (2012).
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242 (2013).
Mole, D. R. Iron homeostasis and its interaction with prolyl hydroxylases. Antioxid. Redox Signal. 12, 445–458 (2010).
Chouchani, E. T. et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515, 431–435 (2014).
Brunelle, J. K. et al. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 1, 409–414 (2005).
Mahon, P. C., Hirota, K. & Semenza, G. L. FIH-1: a novel protein that interacts with HIF-1α and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 15, 2675–2686 (2001).
Jaakkola, P. et al. Targeting of HIF-α to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 (2001).
Imtiyaz, H. Z. et al. Hypoxia-inducible factor 2α regulates macrophage function in mouse models of acute and tumor inflammation. J. Clin. Invest. 120, 2699–2714 (2010).
Piccolo, E. B. et al. Hypoxia-inducible factor-2α enhances neutrophil survival to promote cardiac injury following myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 327, H1230–H1243 (2024).
Heikkila, M., Pasanen, A., Kivirikko, K. I. & Myllyharju, J. Roles of the human hypoxia-inducible factor (HIF)-3α variants in the hypoxia response. Cell. Mol. Life Sci. 68, 3885–3901 (2011).
Maxwell, P. H. et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 (1999).
Wenger, R. H., Stiehl, D. P. & Camenisch, G. Integration of oxygen signaling at the consensus HRE. Sci. STKE 2005, re12 (2005).
Koivunen, P., Hirsila, M., Gunzler, V., Kivirikko, K. I. & Myllyharju, J. Catalytic properties of the asparaginyl hydroxylase (FIH) in the oxygen sensing pathway are distinct from those of its prolyl 4-hydroxylases. J. Biol. Chem. 279, 9899–9904 (2004).
Gao, R. Y. et al. Hypoxia-inducible factor-2α reprograms liver macrophages to protect against acute liver injury through the production of interleukin-6. Hepatology 71, 2105–2117 (2020).
Li, L., et al. Searching for molecular hypoxia sensors among oxygen-dependent enzymes. eLife 12, e87705 (2023).
Batie, M. et al. Hypoxia induces rapid changes to histone methylation and reprograms chromatin. Science 363, 1222–1226 (2019).
Wu, H. & Zhang, Y. Reversing DNA methylation: mechanisms, genomics, and biological functions. Cell 156, 45–68 (2014).
Chakraborty, A. A. et al. Histone demethylase KDM6A directly senses oxygen to control chromatin and cell fate. Science 363, 1217–1222 (2019).
Tahiliani, M. et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324, 930–935 (2009).
Tarhonskaya, H. et al. Investigating the contribution of the active site environment to the slow reaction of hypoxia-inducible factor prolyl hydroxylase domain 2 with oxygen. Biochem. J. 463, 363–372 (2014).
Thienpont, B. et al. Tumour hypoxia causes DNA hypermethylation by reducing TET activity. Nature 537, 63–68 (2016).
Laukka, T. et al. Fumarate and succinate regulate expression of hypoxia-inducible genes via TET enzymes. J. Biol. Chem. 291, 4256–4265 (2016).
Campbell, E. L. et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity 40, 66–77 (2014).
Kominsky, D. J., Campbell, E. L. & Colgan, S. P. Metabolic shifts in immunity and inflammation. J. Immunol. 184, 4062–4068 (2010).
Holmstrom, K. M. & Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 15, 411–421 (2014).
Burn, G. L., Foti, A., Marsman, G., Patel, D. F. & Zychlinsky, A. The neutrophil. Immunity 54, 1377–1391 (2021).
Semenza, G. L., Roth, P. H., Fang, H. M. & Wang, G. L. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem. 269, 23757–23763 (1994).
Walmsley, S. R. et al. Hypoxia-induced neutrophil survival is mediated by HIF-1α-dependent NF-κB activity. J. Exp. Med. 201, 105–115 (2005).
Hoenderdos, K. et al. Hypoxia upregulates neutrophil degranulation and potential for tissue injury. Thorax 71, 1030–1038 (2016).
Sadiku, P. et al. Prolyl hydroxylase 2 inactivation enhances glycogen storage and promotes excessive neutrophilic responses. J. Clin. Invest. 127, 3407–3420 (2017).
Pescador, N., et al. Hypoxia promotes glycogen accumulation through hypoxia inducible factor (HIF)-mediated induction of glycogen synthase 1. PLoS ONE 5, e9644 (2010).
Sadiku, P. et al. Neutrophils fuel effective immune responses through gluconeogenesis and glycogenesis. Cell Metab. 33, 411–423 (2021).
Watts, E. R., et al. Hypoxia drives murine neutrophil protein scavenging to maintain central carbon metabolism. J. Clin. Invest. 131, e134073 (2021).
Cramer, T. et al. HIF-1α is essential for myeloid cell-mediated inflammation. Cell 112, 645–657 (2003).
Mancino, A. et al. Divergent effects of hypoxia on dendritic cell functions. Blood 112, 3723–3734 (2008).
Bosco, M. C. et al. Hypoxia modulates the gene expression profile of immunoregulatory receptors in human mature dendritic cells: identification of TREM-1 as a novel hypoxic marker in vitro and in vivo. Blood 117, 2625–2639 (2011).
Cummins, E. P. et al. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology 134, 156–165 (2008).
Robinson, A. et al. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology 134, 145–155 (2008).
Lee, J. W., Ko, J., Ju, C. & Eltzschig, H. K. Hypoxia signaling in human diseases and therapeutic targets. Exp. Mol. Med. 51, 1–13 (2019).
Bobrow, B., et al. Identification of HIF1A as a therapeutic target during SARS-CoV-2-associated lung injury. JCI Insight 10, e191463 (2025).
Pral, L. P., Fachi, J. L., Correa, R. O., Colonna, M. & Vinolo, M. A. R. Hypoxia and HIF-1 as key regulators of gut microbiota and host interactions. Trends Immunol. 42, 604–621 (2021).
Koeppen, M. et al. Hypoxia-inducible factor 2-α-dependent induction of amphiregulin dampens myocardial ischemia–reperfusion injury. Nat. Commun. 9, 816 (2018).
Ruan, W. et al. BMAL1–HIF2A heterodimer modulates circadian variations of myocardial injury. Nature 641, 1017–1028 (2025).
Eltzschig, H. K., Sitkovsky, M. V. & Robson, S. C. Purinergic signaling during inflammation. N. Engl. J. Med. 367, 2322–2333 (2012).
Colgan, S. P. & Eltzschig, H. K. Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery. Annu. Rev. Physiol. 74, 153–175 (2012).
Eltzschig, H. K. et al. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ. Res. 99, 1100–1108 (2006).
Synnestvedt, K. et al. Ecto-5’-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J. Clin. Invest. 110, 993–1002 (2002).
Eltzschig, H. K. et al. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood 113, 224–232 (2009).
Eckle, T. et al. Identification of ectonucleotidases CD39 and CD73 in innate protection during acute lung injury. J. Immunol. 178, 8127–8137 (2007).
Kong, T., Westerman, K. A., Faigle, M., Eltzschig, H. K. & Colgan, S. P. HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J. 20, 2242–2250 (2006).
Eckle, T. et al. Identification of hypoxia-inducible factor HIF-1A as transcriptional regulator of the A2B adenosine receptor during acute lung injury. J. Immunol. 192, 1249–1256 (2014).
Rosenberger, P. et al. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat. Immunol. 10, 195–202 (2009).
Ly, N. P. et al. Netrin-1 inhibits leukocyte migration in vitro and in vivo. Proc. Natl Acad. Sci. USA 102, 14729–14734 (2005).
Ramkhelawon, B. et al. Hypoxia induces netrin-1 and Unc5b in atherosclerotic plaques: mechanism for macrophage retention and survival. Arterioscler. Thromb. Vasc. Biol. 33, 1180–1188 (2013).
Berg, N. K. et al. Hypoxia-inducible factor-dependent induction of myeloid-derived netrin-1 attenuates natural killer cell infiltration during endotoxin-induced lung injury. FASEB J. 35, e21334 (2021).
Heck-Swain, K. L. et al. Myeloid hypoxia-inducible factor HIF1A provides cardio-protection during ischemia and reperfusion via induction of netrin-1. Front. Cardiovasc. Med. 9, 970415 (2022).
Buckley, C. D., Gilroy, D. W., Serhan, C. N., Stockinger, B. & Tak, P. P. The resolution of inflammation. Nat. Rev. Immunol. 13, 59–66 (2013).
Liu, S. et al. The evolution and heterogeneity of neutrophils in cancers: origins, subsets, functions, orchestrations and clinical applications. Mol. Cancer 22, 148 (2023).
Luo, B. et al. Phagocyte respiratory burst activates macrophage erythropoietin signalling to promote acute inflammation resolution. Nat. Commun. 7, 12177 (2016).
Basil, M. C. & Levy, B. D. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 16, 51–67 (2016).
Norris, P. C., Libreros, S. & Serhan, C. N. Resolution metabolomes activated by hypoxic environment. Sci. Adv. 5, eaax4895 (2019).
Higgins, D. F. et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J. Clin. Invest. 117, 3810–3820 (2007).
Younesi, F. S., Miller, A. E., Barker, T. H., Rossi, F. M. V. & Hinz, B. Fibroblast and myofibroblast activation in normal tissue repair and fibrosis. Nat. Rev. Mol. Cell Biol. 25, 617–638 (2024).
Siddiqui, A. et al. Differential effects of oxygen on human dermal fibroblasts: acute versus chronic hypoxia. Wound Repair Regen. 4, 211–218 (1996).
Yan, S., Li, M., Liu, B., Ma, Z. & Yang, Q. Neutrophil extracellular traps and pulmonary fibrosis: an update. J. Inflamm. 20, 2 (2023).
Chua, F. et al. Mice lacking neutrophil elastase are resistant to bleomycin-induced pulmonary fibrosis. Am. J. Pathol. 170, 65–74 (2007).
Rodriguez-Espinosa, O., Rojas-Espinosa, O., Moreno-Altamirano, M. M., Lopez-Villegas, E. O. & Sanchez-Garcia, F. J. Metabolic requirements for neutrophil extracellular traps formation. Immunology 145, 213–224 (2015).
Frangogiannis, N. Transforming growth factor-β in tissue fibrosis. J. Exp. Med. 217, e20190103 (2020).
Ikeda, N., et al. Emergence of immunoregulatory Ym1+Ly6Chi monocytes during recovery phase of tissue injury. Sci. Immunol. 3, eaat0207 (2018).
Ingersoll, M. A., Platt, A. M., Potteaux, S. & Randolph, G. J. Monocyte trafficking in acute and chronic inflammation. Trends Immunol. 32, 470–477 (2011).
Jiang, Y., et al. Macrophages in organ fibrosis: from pathogenesis to therapeutic targets. Cell Death Discov. 10, 487 (2024).
Corcoran, S. E. & O’Neill, L. A. HIF1α and metabolic reprogramming in inflammation. J. Clin. Invest. 126, 3699–3707 (2016).
Wang, Y. et al. Hypoxia induces M2 macrophages to express VSIG4 and mediate cardiac fibrosis after myocardial infarction. Theranostics 13, 2192–2209 (2023).
Beneke, A. et al. Loss of PHD3 in myeloid cells dampens the inflammatory response and fibrosis after hind-limb ischemia. Cell Death Dis. 8, e2976 (2017).
Misharin, A. V. et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med. 214, 2387–2404 (2017).
Chakraborty, M. et al. Mechanical stiffness controls dendritic cell metabolism and function. Cell Rep. 34, 108609 (2021).
Stefania, K., Ashok, K. K., Geena, P. V., Katarina, P. & Isak, D. TMAO enhances TNF-α mediated fibrosis and release of inflammatory mediators from renal fibroblasts. Sci. Rep. 14, 9070 (2024).
Odell, I. D. et al. Epiregulin is a dendritic cell-derived EGFR ligand that maintains skin and lung fibrosis. Sci. Immunol. 7, eabq6691 (2022).
Dvorak, H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 315, 1650–1659 (1986).
Thomlinson, R. H. Hypoxia and tumours. J. Clin. Pathol. Suppl. (R. Coll. Pathol.) 11, 105–113 (1977).
Paredes, F., Williams, H. C. & San Martin, A. Metabolic adaptation in hypoxia and cancer. Cancer Lett. 502, 133–142 (2021).
Yamauchi, M., Barker, T. H., Gibbons, D. L. & Kurie, J. M. The fibrotic tumor stroma. J. Clin. Invest. 128, 16–25 (2018).
Ding, X. C., et al. The relationship between expression of PD-L1 and HIF-1α in glioma cells under hypoxia. J. Hematol. Oncol. 14, 92 (2021).
Franco, F., Jaccard, A., Romero, P., Yu, Y. R. & Ho, P. C. Metabolic and epigenetic regulation of T-cell exhaustion. Nat. Metab. 2, 1001–1012 (2020).
Ho, P. C. et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell 162, 1217–1228 (2015).
Wang, H. et al. CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat. Immunol. 21, 298–308 (2020).
Di Conza, G. et al. Tumor-induced reshuffling of lipid composition on the endoplasmic reticulum membrane sustains macrophage survival and pro-tumorigenic activity. Nat. Immunol. 22, 1403–1415 (2021).
Tsai, C. H. et al. Immunoediting instructs tumor metabolic reprogramming to support immune evasion. Cell Metab. 35, 118–133 (2023).
Sagiv, J. Y. et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 10, 562–573 (2015).
Manz, M. G. & Boettcher, S. Emergency granulopoiesis. Nat. Rev. Immunol. 14, 302–314 (2014).
Ng, M. S. F. et al. Deterministic reprogramming of neutrophils within tumors. Science 383, eadf6493 (2024).
Singhal, R. et al. Disruption of hypoxia-inducible factor-2α in neutrophils decreases colitis-associated colon cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 326, G53–G66 (2024).
Quin, C., et al. Neutrophil-mediated innate immune resistance to bacterial pneumonia is dependent on Tet2 function. J. Clin. Invest. 134, e171002 (2024).
Fan, C. et al. Hypoxia promotes the tolerogenic phenotype of plasmacytoid dendritic cells in head and neck squamous cell carcinoma. Cancer Med. 11, 922–930 (2022).
Jantsch, J. et al. Hypoxia and hypoxia-inducible factor-1 α modulate lipopolysaccharide-induced dendritic cell activation and function. J. Immunol. 180, 4697–4705 (2008).
Traversari, C., Sozzani, S., Steffensen, K. R. & Russo, V. LXR-dependent and -independent effects of oxysterols on immunity and tumor growth. Eur. J. Immunol. 44, 1896–1903 (2014).
Jhunjhunwala, S., Hammer, C. & Delamarre, L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer 21, 298–312 (2021).
Wenes, M. et al. Macrophage metabolism controls tumor blood vessel morphogenesis and metastasis. Cell Metab. 24, 701–715 (2016).
Su, P. et al. Enhanced lipid accumulation and metabolism are required for the differentiation and activation of tumor-associated macrophages. Cancer Res. 80, 1438–1450 (2020).
Xu, Z. et al. Scavenger receptor CD36 in tumor-associated macrophages promotes cancer progression by dampening type-I IFN signaling. Cancer Res. 85, 462–476 (2025).
Pathria, P., Louis, T. L. & Varner, J. A. Targeting tumor-associated macrophages in cancer. Trends Immunol. 40, 310–327 (2019).
Liu, P. S. et al. CD40 signal rewires fatty acid and glutamine metabolism for stimulating macrophage anti-tumorigenic functions. Nat. Immunol. 24, 452–462 (2023).
Liu, P. S. et al. α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol. 18, 985–994 (2017).
Yuen, C. M. Hyperbaric oxygen therapy as a novel approach to modulating macrophage polarization for the treatment of glioblastoma. Biomedicines 12, 1383 (2024).
Kietzmann, T. Metabolic zonation of the liver: the oxygen gradient revisited. Redox Biol. 11, 622–630 (2017).
Balmer, M. L., et al. The liver may act as a firewall mediating mutualism between the host and its gut commensal microbiota. Sci. Transl. Med. 6, 237ra266 (2014).
Sleyster, E. C. & Knook, D. L. Relation between localization and function of rat liver Kupffer cells. Lab. Invest. 47, 484–490 (1982).
Yuan, X., Ruan, W., Bobrow, B., Carmeliet, P. & Eltzschig, H. K. Targeting hypoxia-inducible factors: therapeutic opportunities and challenges. Nat. Rev. Drug Discov. 23, 175–200 (2024).
Kapitsinou, P. P. et al. Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia. Blood 116, 3039–3048 (2010).
Nagashima, R., Ishikawa, H., Kuno, Y., Kohda, C. & Iyoda, M. HIF–PHD inhibitor regulates the function of group2 innate lymphoid cells and polarization of M2 macrophages. Sci. Rep. 13, 1867 (2023).
Hirota, K. HIF-α prolyl hydroxylase inhibitors and their implications for biomedicine: a comprehensive review. Biomedicines 9, 468 (2021).
Zeng, M. et al. The HIF-1 antagonist acriflavine: visualization in retina and suppression of ocular neovascularization. J. Mol. Med. 95, 417–429 (2017).
Cowman, S. J. & Koh, M. Y. Revisiting the HIF switch in the tumor and its immune microenvironment. Trends Cancer 8, 28–42 (2022).
Hellwig-Burgel, T., Rutkowski, K., Metzen, E., Fandrey, J. & Jelkmann, W. Interleukin-1β and tumor necrosis factor-α stimulate DNA binding of hypoxia-inducible factor-1. Blood 94, 1561–1567 (1999).
Mirchandani, A. S., Sanchez-Garcia, M. A. & Walmsley, S. R. How oxygenation shapes immune responses: emerging roles for physioxia and pathological hypoxia. Nat. Rev. Immunol. 25, 161–177 (2024).
Acknowledgements
P.-C.H. is supported by Ludwig Cancer Research, the Swiss National Science Foundation, the Cancer Research Institute (Lloyd J. Old STAR award), the Swiss Cancer League and the Helmut Horten Stiftung.
Author information
Authors and Affiliations
Contributions
J.T.L. and P.-C.H. contributed to the conceptualization. J.T.L., Y.K., M.M. and P.-C.H. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
P.-C.H. is a cofounder of Pilatus Biosciences.
Peer review
Peer review information
Nature Immunology thanks Navdeep Chandel and the other anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Ioana Staicu, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Low, J.T., Kim, Y., Matsushita, M. et al. Role of oxygen sensing and hypoxia-inducible factors in orchestrating innate immune responses. Nat Immunol 26, 2138–2147 (2025). https://doi.org/10.1038/s41590-025-02317-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41590-025-02317-1