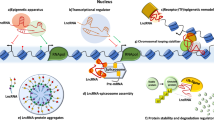
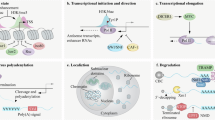
Abstract
Genes encoding long noncoding RNAs (lncRNAs) are intimately involved in mammalian immunity. Here we review recent knowledge of how lncRNAs regulate immune cell specification and function. Beyond canonical roles in nuclear architecture, chromatin modification and posttranscriptional regulation, lncRNA regulation of metabolic pathways, antigenic extracellular lncRNA ribonucleoprotein complexes and glycosylated noncoding RNAs on the cell surface have emerged as newly recognized regulatory mechanisms. In the immune system, specific lncRNAs control lineage determination during hematopoiesis as well as immune cell activation and function during immune responses, while lncRNA dysregulation is associated with immune-related diseases. In particular, we highlight how a female-specific lncRNA XIST promotes female-biased autoimmunity. Finally, we discuss emerging technologies in high-throughput functional genomics, human genetics, molecular interaction mapping, artificial intelligence and synthetic biology that are accelerating our understanding of lncRNA biology in immunity and beyond.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Iyer, M. K. et al. The landscape of long noncoding RNAs in the human transcriptome. Nat. Genet. 47, 199–208 (2015).
Kaur, G. et al. GENCODE: massively expanding the lncRNA catalog through capture long-read RNA sequencing. Preprint at bioRxiv https://doi.org/10.1101/2024.10.29.620654 (2024).
Mattick, J. S. et al. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 24, 430–447 (2023).
Quinn, J. J. et al. Rapid evolutionary turnover underlies conserved lncRNA-genome interactions. Genes Dev. 30, 191–207 (2016).
Carlevaro-Fita, J. et al. Ancient exapted transposable elements promote nuclear enrichment of human long noncoding RNAs. Genome Res. 29, 208–222 (2019).
Morrison, T. A. et al. Evolving views of long noncoding RNAs and epigenomic control of lymphocyte state and memory. Cold Spring Harb. Perspect. Biol. 14, a037952 (2022).
Chen, Y. G., Satpathy, A. T. & Chang, H. Y. Gene regulation in the immune system by long noncoding RNAs. Nat. Immunol. 18, 962–972 (2017).
Peltier, D. C., Roberts, A. & Reddy, P. LNCing RNA to immunity. Trends Immunol. 43, 478–495 (2022).
Fanucchi, S. et al. Immune genes are primed for robust transcription by proximal long noncoding RNAs located in nuclear compartments. Nat. Genet. 51, 138–150 (2019).
Hacisuleyman, E. et al. Topological organization of multichromosomal regions by the long intergenic noncoding RNA Firre. Nat. Struct. Mol. Biol. 21, 198–206 (2014).
Lewandowski, J. P. et al. The Firre locus produces a trans-acting RNA molecule that functions in hematopoiesis. Nat. Commun. 10, 5137 (2019).
Imamura, K. et al. Long noncoding RNA NEAT1-dependent SFPQ relocation from promoter region to paraspeckle mediates IL8 expression upon immune stimuli. Mol. Cell 53, 393–406 (2014).
Azam, S. et al. The early macrophage response to pathogens requires dynamic regulation of the nuclear paraspeckle. Proc. Natl Acad. Sci. USA 121, e2312587121 (2024).
Carpenter, S. et al. A long noncoding RNA mediates both activation and repression of immune response genes. Science 341, 789–792 (2013).
Yang, S. et al. WDR82-binding long noncoding RNA lncEry controls mouse erythroid differentiation and maturation. J. Exp. Med. 219, e20211688 (2022).
Yang, T., Ou, J. & Yildirim, E. Xist exerts gene-specific silencing during XCI maintenance and impacts lineage-specific cell differentiation and proliferation during hematopoiesis. Nat. Commun. 13, 4464 (2022).
Atianand, M. K. et al. A long noncoding RNA lincRNA-EPS acts as a transcriptional brake to restrain inflammation. Cell 165, 1672–1685 (2016).
Gomez, J. A. et al. The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-γ locus. Cell 152, 743–754 (2013).
Kotzin, J. J. et al. The long non-coding RNA Morrbid regulates Bim and short-lived myeloid cell lifespan. Nature 537, 239–243 (2016).
Wheeler, B. D. et al. The lncRNA Malat1 inhibits miR-15/16 to enhance cytotoxic T cell activation and memory cell formation. Elife 12, RP87900 (2023).
Elguindy, M. M. & Mendell, J. T. NORAD-induced Pumilio phase separation is required for genome stability. Nature 595, 303–308 (2021).
Wang, P. et al. The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science 344, 310–313 (2014).
Huang, D. et al. NKILA lncRNA promotes tumor immune evasion by sensitizing T cells to activation-induced cell death. Nat. Immunol. 19, 1112–1125 (2018).
Sharma, S. et al. Dephosphorylation of the nuclear factor of activated T cells (NFAT) transcription factor is regulated by an RNA-protein scaffold complex. Proc. Natl Acad. Sci. USA 108, 11381–11386 (2011).
Rapicavoli, N. A. et al. A mammalian pseudogene lncRNA at the interface of inflammation and anti-inflammatory therapeutics. Elife 2, e00762 (2013).
Wang, P., Xu, J., Wang, Y. & Cao, X. An interferon-independent lncRNA promotes viral replication by modulating cellular metabolism. Science 358, 1051–1055 (2017).
Sang, L. et al. Mitochondrial long non-coding RNA GAS5 tunes TCA metabolism in response to nutrient stress. Nat. Metab. 3, 90–106 (2021).
Liu, J. et al. CCR7 chemokine receptor-inducible lnc-Dpf3 restrains dendritic cell migration by inhibiting HIF-1α-mediated glycolysis. Immunity 50, 600–615 (2019).
Shmuel-Galia, L. et al. The lncRNA HOXA11os regulates mitochondrial function in myeloid cells to maintain intestinal homeostasis. Cell Metab. 35, 1441–1456 (2023).
Huang, N. et al. Natural display of nuclear-encoded RNA on the cell surface and its impact on cell interaction. Genome Biol. 21, 225 (2020).
Flynn, R. A. et al. Small RNAs are modified with N-glycans and displayed on the surface of living cells. Cell 184, 3109–3124 (2021).
Jiang, X. et al. Small non-coding RNAs encapsulating mammalian cells fuel innate immunity. Preprint at bioRxiv https://doi.org/10.1101/2025.04.07.647669 (2025).
Xie, Y. et al. The modified RNA base acp3U is an attachment site for N-glycans in glycoRNA. Cell 187, 5228–5237 (2024).
Targeting a cell surface RNA-binding protein driving acute myeloid leukemia. Nat. Biotechnol. https://doi.org/10.1038/s41587-025-02695-9 (2025).
Graziano, V. R. et al. RNA N-glycosylation enables immune evasion and homeostatic efferocytosis. Nature 645, 784–792 (2025).
Zhang, N. et al. Cell surface RNAs control neutrophil recruitment. Cell 187, 846–860 (2024).
Chu, C. et al. Systematic discovery of Xist RNA binding proteins. Cell 161, 404–416 (2015).
Núñez-Martínez, H. N. & Recillas-Targa, F. Emerging functions of lncRNA Loci beyond the transcript Itself. Int. J. Mol. Sci. 23, 6258 (2022).
Mowel, W. K. et al. Group 1 innate lymphoid cell lineage identity is determined by a cis-regulatory element marked by a long non-coding RNA. Immunity 47, 435–449 (2017).
Isoda, T. et al. Non-coding transcription instructs chromatin folding and compartmentalization to dictate enhancer-promoter communication and T cell fate. Cell 171, 103–119 (2017).
Abarrategui, I. & Krangel, M. S. Noncoding transcription controls downstream promoters to regulate T-cell receptor alpha recombination. EMBO J. 26, 4380–4390 (2007).
Rothschild, G. et al. Noncoding RNA transcription alters chromosomal topology to promote isotype-specific class switch recombination. Sci. Immunol. 5, eaay5864 (2020).
Ruiz-Orera, J., Messeguer, X., Subirana, J. A. & Alba, M. M. Long non-coding RNAs as a source of new peptides. Elife 3, e03523 (2014).
Carlevaro-Fita, J., Rahim, A., Guigó, R., Vardy, L. A. & Johnson, R. Cytoplasmic long noncoding RNAs are frequently bound to and degraded at ribosomes in human cells. RNA 22, 867–882 (2016).
Ingolia, N. T. et al. Ribosome profiling reveals pervasive translation outside of annotated protein-coding genes. Cell Rep. 8, 1365–1379 (2014).
Tang, S. et al. A lncRNA Dleu2-encoded peptide relieves autoimmunity by facilitating Smad3-mediated Treg induction. EMBO Rep. 25, 1208–1232 (2024).
Niu, L. et al. A micropeptide encoded by lncRNA MIR155HG suppresses autoimmune inflammation via modulating antigen presentation. Sci. Adv. 6, eaaz2059 (2020).
Barczak, W. et al. Long non-coding RNA-derived peptides are immunogenic and drive a potent anti-tumour response. Nat. Commun. 14, 1078 (2023).
Liu, B. et al. Long noncoding RNA lncKdm2b is required for ILC3 maintenance by initiation of Zfp292 expression. Nat. Immunol. 18, 499–508 (2017).
Zemmour, D., Pratama, A., Loughhead, S. M., Mathis, D. & Benoist, C. Flicr, a long noncoding RNA, modulates Foxp3 expression and autoimmunity. Proc. Natl Acad. Sci. USA 114, E3472–E3480 (2017).
Chen, Y. et al. lncRNA-GM targets Foxo1 to promote T cell-mediated autoimmunity. Sci. Adv. 8, eabn9181 (2022).
Vollmers, A. C. et al. A conserved long noncoding RNA, GAPLINC, modulates the immune response during endotoxic shock. Proc. Natl Acad. Sci. USA 118, e2016648118 (2021).
Xu, J. et al. IRF3-binding lncRNA-ISIR strengthens interferon production in viral infection and autoinflammation. Cell Rep. 37, 109926 (2021).
Liu, J. et al. The IRENA lncRNA converts chemotherapy-polarized tumor-suppressing macrophages to tumor-promoting phenotypes in breast cancer. Nat. Cancer 2, 457–473 (2021).
Wang, S. et al. An NF-κB-driven lncRNA orchestrates colitis and circadian clock. Sci. Adv. 6, eabb5202 (2020).
Lin, H. et al. The long noncoding RNA Lnczc3h7a promotes a TRIM25-mediated RIG-I antiviral innate immune response. Nat. Immunol. 20, 812–823 (2019).
Jiang, M. et al. Self-recognition of an inducible host lncRNA by RIG-I feedback restricts innate immune response. Cell 173, 906–919 (2018).
Imam, H., Bano, A. S., Patel, P., Holla, P. & Jameel, S. The lncRNA NRON modulates HIV-1 replication in a NFAT-dependent manner and is differentially regulated by early and late viral proteins. Sci. Rep. 5, 8639 (2015).
Chao, T. -C. et al. The long noncoding RNA HEAL regulates HIV-1 replication through epigenetic regulation of the HIV-1 promoter. mBio 10, e02016–e02019 (2019).
Cao, Y. et al. An interferon-stimulated long non-coding RNA USP30-AS1 as an immune modulator in influenza A virus infection. PLoS Pathog. 21, e1012854 (2025).
Wang, Y. et al. Long noncoding RNA derived from CD244 signaling epigenetically controls CD8+ T-cell immune responses in tuberculosis infection. Proc. Natl Acad. Sci. USA 112, E3883–E3892 (2015).
Gcanga, L. et al. Host-directed targeting of LincRNA-MIR99AHG suppresses intracellular growth of Mycobacterium tuberculosis. Nucleic Acid Ther. 32, 421–437 (2022).
Han, X. et al. LncRNA PTPRE-AS1 modulates M2 macrophage activation and inflammatory diseases by epigenetic promotion of PTPRE. Sci. Adv. 5, eaax9230 (2019).
Castellanos-Rubio, A. et al. A long noncoding RNA associated with susceptibility to celiac disease. Science 352, 91–95 (2016).
Gonzalez-Moro, I. et al. The T1D-associated lncRNA Lnc13 modulates human pancreatic β cell inflammation by allele-specific stabilization of STAT1 mRNA. Proc. Natl Acad. Sci. USA 117, 9022–9031 (2020).
Yang, Z. et al. Promotion of TLR7-MyD88-dependent inflammation and autoimmunity in mice through stem-loop changes in Lnc-Atg16l1. Nat. Commun. 15, 10224 (2024).
Klein, S. L. & Flanagan, K. L. Sex differences in immune responses. Nat. Rev. Immunol. 16, 626–638 (2016).
Yu, B. et al. B cell-specific XIST complex enforces X-inactivation and restrains atypical B cells. Cell 184, 1790–1803 (2021).
Dou, D. R. et al. Xist ribonucleoproteins promote female sex-biased autoimmunity. Cell 187, 733–749 (2024).
Gartler, S. M. & Riggs, A. D. Mammalian X-chromosome inactivation. Annu. Rev. Genet. 17, 155–190 (1983).
Plath, K., Mlynarczyk-Evans, S., Nusinow, D. A. & Panning, B. Xist RNA and the mechanism of X chromosome inactivation. Annu. Rev. Genet. 36, 233–278 (2002).
Loda, A., Collombet, S. & Heard, E. Gene regulation in time and space during X-chromosome inactivation. Nat. Rev. Mol. Cell Biol. 23, 231–249 (2022).
Subramanian, S. et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc. Natl Acad. Sci. USA 103, 9970–9975 (2006).
Christensen, S. R. et al. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity 25, 417–428 (2006).
Lau, L. et al. An essential role for TASL in mouse autoimmune pathogenesis and Toll-like receptor signaling. Nat. Commun. 16, 968 (2025).
Jenks, S. A. et al. Distinct effector B cells induced by unregulated Toll-like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity 49, 725–739 (2018).
Ricker, E. et al. Altered function and differentiation of age-associated B cells contribute to the female bias in lupus mice. Nat. Commun. 12, 4813 (2021).
Rubtsov, A. V. et al. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c+ B-cell population is important for the development of autoimmunity. Blood 118, 1305–1315 (2011).
Rubtsov, A. V. et al. CD11c-expressing B cells are located at the T cell/B cell border in spleen and are potent APCs. J. Immunol. 195, 71–79 (2015).
Zhang, W. et al. Excessive CD11c+Tbet+ B cells promote aberrant TFH differentiation and affinity-based germinal center selection in lupus. Proc. Natl Acad. Sci. USA 116, 18550–18560 (2019).
Karnell, J. L. et al. Role of CD11c+ T-bet+ B cells in human health and disease. Cell Immunol. 321, 40–45 (2017).
Wang, S. et al. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11chiT-bet+ B cells in SLE. Nat. Commun. 9, 1758 (2018).
Lovell, C. D., Jiwrajka, N., Amerman, H. K., Cancro, M. P. & Anguera, M. C. Xist deletion in B cells results in systemic lupus erythematosus phenotypes. Preprint at bioRxiv https://doi.org/10.1101/2024.05.15.594175 (2024).
Du, Y. et al. Altered X-chromosome inactivation of the TLR7/8 locus and heterogeneity of pDCs in systemic sclerosis. J. Exp. Med. 222, e20231809 (2025).
Brooks, W. H., Satoh, M., Hong, B., Reeves, W. H. & Yang, T. P. Autoantibodies from an SLE patient immunostain the Barr body. Cytogenet. Genome Res. 97, 28–31 (2002).
Hong, B., Reeves, P., Panning, B., Swanson, M. S. & Yang, T. P. Identification of an autoimmune serum containing antibodies against the Barr body. Proc. Natl Acad. Sci. USA 98, 8703–8708 (2001).
Crawford, J. D. et al. The XIST lncRNA is a sex-specific reservoir of TLR7 ligands in SLE. JCI Insight 8, e169344 (2023).
Carter, A. C. et al. Spen links RNA-mediated endogenous retrovirus silencing and X chromosome inactivation. Elife 9, e54508 (2020).
Yu, P. et al. Nucleic acid-sensing Toll-like receptors are essential for the control of endogenous retrovirus viremia and ERV-induced tumors. Immunity 37, 867–879 (2012).
Yan, B. et al. Autoantibody hotspots reveal origin and impact of immunogenic XIST ribonucleoprotein complex. Preprint at bioRxiv https://doi.org/10.1101/2025.01.16.633465 (2025).
Wang, J. et al. Unusual maintenance of X chromosome inactivation predisposes female lymphocytes for increased expression from the inactive X. Proc. Natl Acad. Sci. USA 113, E2029–E2038 (2016).
Pyfrom, S. et al. The dynamic epigenetic regulation of the inactive X chromosome in healthy human B cells is dysregulated in lupus patients. Proc. Natl Acad. Sci. USA 118, e2024624118 (2021).
Hansen, J. E. et al. Targeting cancer with a lupus autoantibody. Sci. Transl. Med. 4, 157ra142 (2012).
Weisbart, R. H. et al. DNA-dependent targeting of cell nuclei by a lupus autoantibody. Sci. Rep. 5, 12022 (2015).
Liu, Y. et al. Genome-wide screening for functional long noncoding RNAs in human cells by Cas9 targeting of splice sites. Nat. Biotechnol. 36, 1203–1210 (2018).
Wang, Y. et al. Genome-wide gain-of-function screening characterized lncRNA regulators for tumor immune response. Sci. Adv. 8, eadd0005 (2022).
Halasz, H. et al. CRISPRi screens identify the lncRNA, LOUP, as a multifunctional locus regulating macrophage differentiation and inflammatory signaling. Proc. Natl Acad. Sci. USA 121, e2322524121 (2024).
Liang, W. -W. et al. Transcriptome-scale RNA-targeting CRISPR screens reveal essential lncRNAs in human cells. Cell 187, 7637–7654 (2024).
Montero, J. J. et al. Genome-scale pan-cancer interrogation of lncRNA dependencies using CasRx. Nat. Methods 21, 584–596 (2024).
S Zibitt, M., Hartford, C. C. R. & Lal, A. Interrogating lncRNA functions via CRISPR/Cas systems. RNA Biol. 18, 2097–2106 (2021).
Pacalin, N. M. et al. Bidirectional epigenetic editing reveals hierarchies in gene regulation. Nat. Biotechnol. 43, 355–368 (2025).
Horlbeck, M. A., Liu, S. J., Chang, H. Y., Lim, D. A. & Weissman, J. S. Fitness effects of CRISPR/Cas9-targeting of long noncoding RNA genes. Nat. Biotechnol. 38, 573–576 (2020).
Deng, Y. et al. Generation of a CRISPR activation mouse that enables modelling of aggressive lymphoma and interrogation of venetoclax resistance. Nat. Commun. 13, 4739 (2022).
Gemberling, M. P. et al. Transgenic mice for in vivo epigenome editing with CRISPR-based systems. Nat. Methods 18, 965–974 (2021).
Neal, J. T. et al. Organoid modeling of the tumor immune microenvironment. Cell 175, 1972–1988 (2018).
Wagar, L. E. et al. Modeling human adaptive immune responses with tonsil organoids. Nat. Med. 27, 125–135 (2021).
Santos, A. J. M. et al. A human autoimmune organoid model reveals IL-7 function in celiac disease. Nature 632, 401–410 (2024).
Dixit, A. et al. Perturb-seq: dissecting molecular circuits with scalable single-cell RNA profiling of pooled genetic screens. Cell 167, 1853–1866 (2016).
Ang, C. E. et al. The novel lncRNA lnc-NR2F1 is pro-neurogenic and mutated in human neurodevelopmental disorders. eLife 8, e41770 (2019).
Ganesh, V. S. et al. Neurodevelopmental disorder caused by deletion of CHASERR, a lncRNA gene. N. Engl. J. Med. 391, 1511–1518 (2024).
Rom, A. et al. Regulation of CHD2 expression by the Chaserr long noncoding RNA gene is essential for viability. Nat. Commun. 10, 5092 (2019).
Miolo, G. et al. Identification of a de novo Xq26.2 microduplication encompassing FIRRE gene in a child with intellectual disability. Diagnostics 10, 1009 (2020).
Allou, L. et al. Non-coding deletions identify Maenli lncRNA as a limb-specific En1 regulator. Nature 592, 93–98 (2021).
Cho, S. W. et al. Promoter of lncRNA gene PVT1 is a tumor-suppressor DNA boundary element. Cell 173, 1398–1412 (2018).
Szafranski, P., Gambin, T., Karolak, J. A., Popek, E. & Stankiewicz, P. Lung-specific distant enhancer cis regulates expression of FOXF1 and lncRNA FENDRR. Hum. Mutat. 42, 694–698 (2021).
Andersen, R. E. et al. Chromosomal structural rearrangements implicate long non-coding RNAs in rare germline disorders. Hum. Genet. 143, 921–938 (2024).
de Goede, O. M. et al. Population-scale tissue transcriptomics maps long non-coding RNAs to complex disease. Cell 184, 2633–2648 (2021).
Ma, H. et al. A lncRNA from an inflammatory bowel disease risk locus maintains intestinal host-commensal homeostasis. Cell Res. 33, 372–388 (2023).
Chu, C., Qu, K., Zhong, F. L., Artandi, S. E. & Chang, H. Y. Genomic maps of long noncoding RNA occupancy reveal principles of RNA-chromatin interactions. Mol. Cell 44, 667–678 (2011).
Simon, M. D. et al. The genomic binding sites of a noncoding RNA. Proc. Natl Acad. Sci. USA 108, 20497–20502 (2011).
Engreitz, J., Lander, E. S. & Guttman, M. RNA antisense purification (RAP) for mapping RNA interactions with chromatin. Methods Mol. Biol. 1262, 183–197 (2015).
Tsue, A. F. et al. Multiomic characterization of RNA microenvironments by oligonucleotide-mediated proximity-interactome mapping. Nat. Methods 21, 2058–2071 (2024).
Wolin, E. et al. SPIDR enables multiplexed mapping of RNA-protein interactions and uncovers a mechanism for selective translational suppression upon cell stress. Cell 188, 5384–5402.e25 (2025).
MAPIT-seq reveals both RBP targets and transcriptome-wide gene expression profiles. Nat. Methods 22, 1768–1769 (2025).
Quinodoz, S. A. et al. RNA promotes the formation of spatial compartments in the nucleus. Cell 184, 5775–5790 (2021).
Wen, X. et al. Single-cell multiplex chromatin and RNA interactions in ageing human brain. Nature 628, 648–656 (2024).
Zhou, Y. et al. The RNA-binding protein RRP1 brakes macrophage one-carbon metabolism to suppress autoinflammation. Nat. Commun. 16, 6880 (2025).
Dodel, M. et al. TREX reveals proteins that bind to specific RNA regions in living cells. Nat. Methods 21, 423–434 (2024).
Yi, H. et al. EcDNA-borne PVT1 fusion stabilizes oncogenic mRNAs. Preprint at bioRxiv https://doi.org/10.1101/2025.04.01.646515 (2025).
Brixi, G. et al. Genome modeling and design across all domains of life with Evo 2. Preprint at bioRxiv https://doi.org/10.1101/2025.02.18.638918 (2025).
Chiang, J. -C., Jiang, J., Newburger, P. E. & Lawrence, J. B. Trisomy silencing by XIST normalizes Down syndrome cell pathogenesis demonstrated for hematopoietic defects in vitro. Nat. Commun. 9, 5180 (2018).
Gupta, K., Czerminski, J. T. & Lawrence, J. B. Trisomy silencing by XIST: translational prospects and challenges. Hum. Genet 143, 843–855 (2024).
Navarro-Cobos, M. J., Morales-Guzman, S. I., Baldry, S. E. L. & Brown, C. J. Derivation of a minimal functional XIST by combining human and mouse interaction domains. Hum. Mol. Genet. 32, 1289–1300 (2023).
Acknowledgements
We thank members of the Chang and Yu labs for discussions. Supported by Stanford RNA Medicine Program (to H.Y.C.) and Scleroderma Research Foundation (to H.Y.C. and B.Y.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
H.Y.C. is a cofounder of Accent Therapeutics, Boundless Bio, Cartography Biosciences and Orbital Therapeutics, and was an advisor of 10x Genomics, Arsenal Bio, Chroma Medicine, Exai Bio and Vida Ventures. H.Y.C. is an employee and stockholder of Amgen as of 16 December 2024. B.Y. has no competing interests.
Peer review
Peer review information
Nature Immunology thanks Katherine Fitzgerald, K. Mark Ansel, Xuetao Cao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Jamie D. K. Wilson, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, B., Chang, H.Y. Long noncoding RNA regulation of immunity. Nat Immunol 27, 16–25 (2026). https://doi.org/10.1038/s41590-025-02355-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41590-025-02355-9