Figure 1
From: Cyclized NDGA modifies dynamic α-synuclein monomers preventing aggregation and toxicity
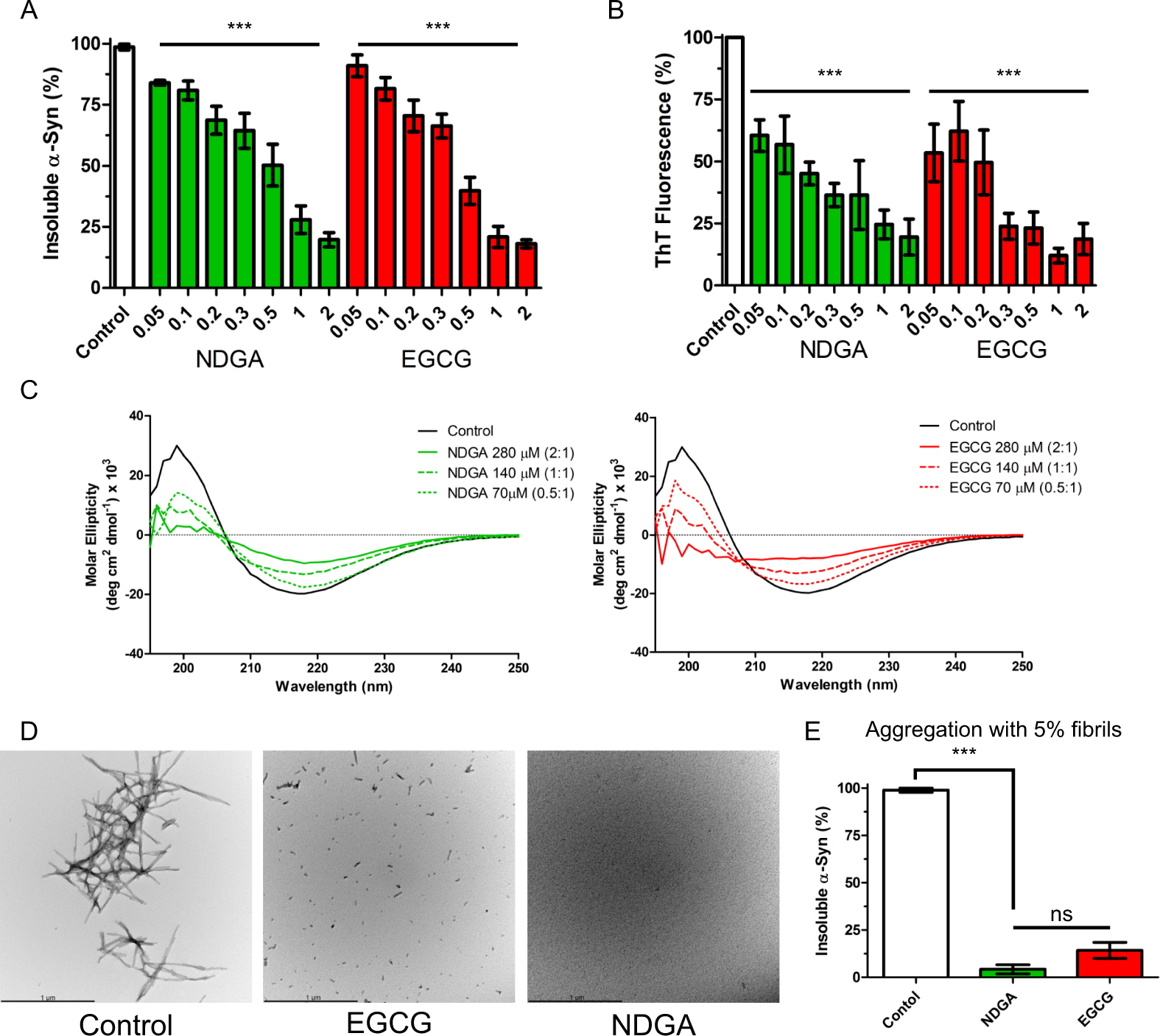
NDGA inhibits recombinant human α-synuclein aggregation. (A) Insoluble α-synuclein present after 7 days aggregation was reduced by NDGA and EGCG in a dose-dependent fashion as compared to solvent control. Recombinant human wildtype α-synuclein (138 µM) was aggregated for 7 days in the presence of EGCG or NDGA at the indicated molar ratios. After aggregation, PBS-insoluble α-synuclein was separated by centrifugation (21k g for 10 min). Soluble and insoluble fractions were boiled in SDS, run by SDS-PAGE, and colloidal stained. α-Synuclein in each fraction was quantified by in-gel densitometry (n = 3–5, ***p < 0.001). (B) Formation of amyloid α-synuclein fibrils quantified by Thioflavin-T was reduced by NDGA and EGCG in a dose-dependent fashion as compared to control after 7 days aggregation (n = 3–5, ***p < 0.001). (C) α-Synuclein beta-sheet secondary structure was reduced by EGCG and NDGA in a dose dependent fashion. Recombinant human wildtype α-synuclein (138 µM) was aggregated for 7 days in the presence of EGCG or NDGA at the indicated molar ratios. Secondary structure was quantified by circular dichroism. (D) Transmission electron microscopy images of α-synuclein aggregates after 3 days aggregation with small molecules at 1:1 molar ratio. (E) Insoluble α-synuclein present after 7 days aggregation in the presence of a 5% fibril seed was reduced by EGCG and NDGA. EGCG or NDGA were present at a 1:1 molar ratio. After aggregation, PBS-insoluble α-synuclein was separated by centrifugation (21k g for 10 min). Soluble and insoluble fractions were boiled in SDS, run by SDS-PAGE, and colloidal stained. α-Synuclein in each fraction was quantified by in-gel densitometry (n = 3, ***p < 0.001).
