Table 4 NiO NPs-catalyzed the synthesis of pyridopyrimidine derivativesa.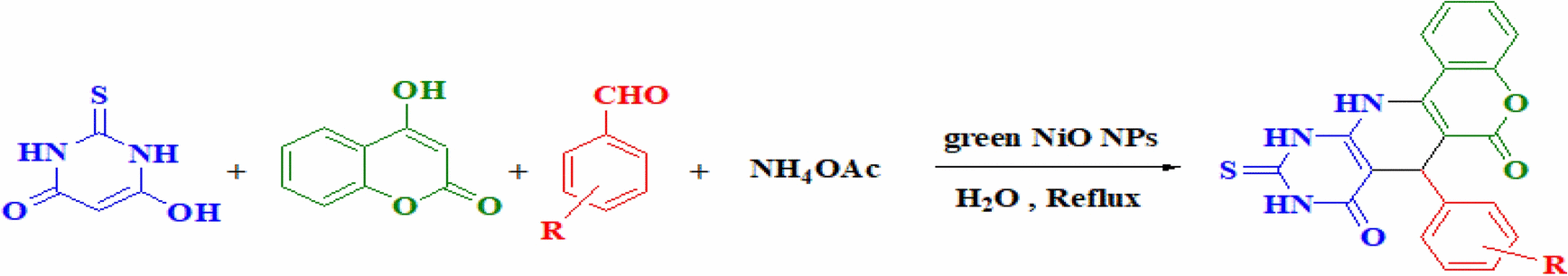
From: Algal magnetic nickel oxide nanocatalyst in accelerated synthesis of pyridopyrimidine derivatives
Entrya | R | Product | Time (min) | TONc | TOFd (min-1) | Yieldb (%) | MP (ºC) (Ref.) | |
|---|---|---|---|---|---|---|---|---|
1 | H |
| 5a | 45 | 17,600 | 391 | 88 | 235–237 |
(236–238)60 | ||||||||
2 | 2-Cl |
| 5b | 47 | 18,400 | 391.49 | 92 | 215–217 |
(218–220)60 | ||||||||
3 | 3-Cl |
| 5c | 50 | 18,000 | 360 | 90 | 220–222 |
(219–220)60 | ||||||||
4 | 4-Cl |
| 5d | 42 | 18,800 | 447.62 | 94 | 246–248 |
(244–246)60 | ||||||||
5 | 2,4-DiCl |
| 5e | 48 | 17,000 | 354.16 | 85 | 227–229 |
(229–230)60 | ||||||||
6 | 2-NO2 |
| 5f | 50 | 17,000 | 360 | 90 | 236–238 |
(235–237)60 | ||||||||
7 | 4-NO2 |
| 5g | 40 | 19,200 | 480 | 96 | 240–242 |
(239–241)60 | ||||||||
8 | 3-OCH3 |
| 5h | 55 | 17,600 | 320 | 88 | 221–223 |
(222–223)60 | ||||||||
9 | 4-CN |
| 5i | 48 | 18,400 | 383.33 | 92 | 245–247 |
(244–245)60 | ||||||||
10 | 4-Br |
| 5j | 55 | 18,000 | 327.27 | 90 | 233–235 |
(231–233)60 |











