Figure 2
From: MarrowCellDLD: a microfluidic method for label-free retrieval of fragile bone marrow-derived cells
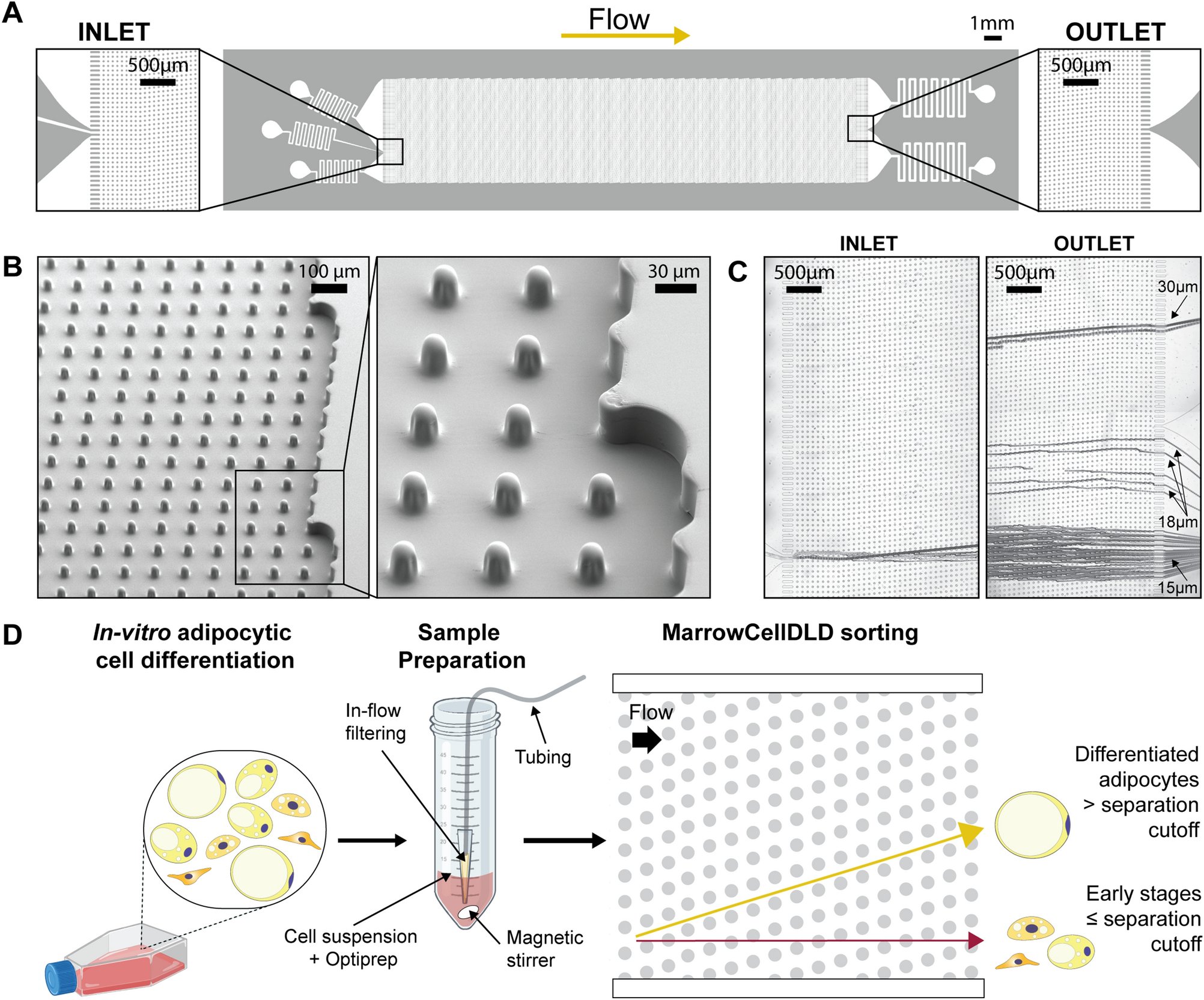
MarrowCellDLD device and operation. (A) MarrowCellDLD chip: insights on inlet and outlet regions terminated with 10 rows of straight pillars respectively before and after the MarrowCellDLD array active region (tilted array). (B) Scanning electron microscopy images of the MarrowCellDLD array chip with a 19 μm separation cutoff (critical size). (C) Inlet and outlet trajectories of polystyrene microbeads of 15, 18, and 20 μm in size transiting a MarrowCellDLD chip with 19 μm critical size. (D) Experimental workflow to sort differentiated adipocytes by MarrowCellDLD. After adipocytic differentiation in vitro, the induced-OP9 sample contains a mixture of progenitor cells, early stages of differentiation, and mature adipocytes. After trypsinization, the cellular sample is suspended in its original culture media supplemented with Optiprep, then placed at the inlet reservoir connected to the tubing responsible for injecting the sample into the MarrowCellDLD device. A custom in-flow filtering system embedded in the tubing ensures the injection of a single-cell suspension, and the sample is continuously stirred to achieve homogeneity. Within the sorting module, mature adipocytes larger than the critical size for separation should be forced to follow the array angle (displacement mode), allowing for their isolation. Conversely, early stages of differentiation and progenitors should move parallel to the flow (zig–zag mode). The two fractions are thus predicted to be physically separated and can be collected at distinct outlets.
