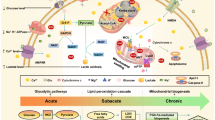
Abstract
While clinical data firmly establish that Peripheral artery disease is linked to a higher incidence and severity of ischemic stroke, the underlying hemodynamics and metabolic mechanisms remain poorly defined. This study aimed to elucidate how chronic limb ischemia remotely exacerbates ischemic stroke outcomes through these mechanisms. Computational fluid dynamics combined with high-resolution MRI was used to quantify cerebral hemodynamics, including wall shear stress (WSS), blood flow velocity, and wall pressure. Integrative omics was applied to analyze molecular changes. Focal cerebral ischemia was induced during chronic hindlimb ischemia to assess stroke outcomes. Our results showed that both WSS and blood flow velocity were significantly decreased in the model group compared with the sham group, particularly in the middle cerebral artery. Chronic hindlimb ischemia exacerbated brain edema, increased infarct volume by 1.5-fold, and worsened neurological deficits. Integrated omics analysis revealed disturbances in glycerophospholipid metabolism and identified phosphate cytidylyltransferase 2 (PCYT2) as a key upregulated protein. Crucially, in vivo functional validation demonstrated that silencing PCYT2 conferred significant neuroprotection, reducing infarct volume and improving neurological outcomes. These findings enhance our understanding of the pathogenesis mechanisms by which chronic limb ischemia aggravates ischemic stroke and provide new insights into its prevention and treatment.
Similar content being viewed by others
Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
Pu, L. et al. Projected global trends in ischemic stroke incidence, deaths and disability-adjusted life years from 2020 to 2030. Stroke 54(5), 1330–1339 (2023).
Mandaglio-Collados, D., Marin, F. & Rivera-Caravaca, J. M. Peripheral artery disease: Update on etiology, pathophysiology, diagnosis and treatment. Med. Clin. (Barc). 161(8), 344–350. https://doi.org/10.1016/j.medcli.2023.06.005 (2023).
Cheshire, B. L., Messeder, S. J., Pepper, C. J., Beishon L. C., Sayers R. D., Houghton, J. S., Association of cognitive impairment and peripheral artery disease (PAD): A systematic review. In Vascular medicine. (2025): 1358863X251336736.
Song, J. Y. & Kwon, S. U. Intracranial atherosclerotic stenosis. Cerebrovasc Dis. Extra. 15(1), 62–67. https://doi.org/10.1159/000543356 (2025).
Yu, L. et al. Remote limb ischemic postconditioning inhibits microglia pyroptosis by modulating HGF after acute ischemia stroke. Bioeng Transl. Med. 8(6), e10590. https://doi.org/10.1002/btm2.10590 (2023).
Valsecchi, V. et al. The hypoxia sensitive metal transcription factor MTF-1 activates NCX1 brain promoter and participates in remote postconditioning neuroprotection in stroke. Cell Death Dis. 12(5), 423. https://doi.org/10.1038/s41419-021-03705-9 (2021).
Cuomo, O. et al. Systemic administration of blood-derived exosomes induced by remote ischemic post-conditioning, by delivering a specific cluster of miRNAs, ameliorates ischemic damage and neurological function. J. Cereb. Blood Flow Metab. 44(12), 1459–1471. https://doi.org/10.1177/0271678X241270284 (2024).
Saglietto, A. et al. Role of the vessel morphology on the lenticulostriate arteries hemodynamics during atrial fibrillation: A CFD-based multivariate regression analysis. Comput. Method. Programs Biomed. 254, 108303. https://doi.org/10.1016/j.cmpb.2024.108303 (2024).
Chen, Y. et al. Non-invasive assessment of intracranial wall shear stress using high-resolution magnetic resonance imaging in combination with computational fluid dynamics technique. Fundam. Res. 2(2), 329–334. https://doi.org/10.1016/j.fmre.2021.09.019 (2022).
Hattori, Y. et al. High middle cerebral artery wall shear stress in branch atheromatous disease: A computational fluid dynamics analysis. J. Atheroscler. Thromb. https://doi.org/10.5551/jat.65439 (2025).
Zhou, M. et al. Wall shear stress and its role in atherosclerosis. Front. Cardiovascular Med. 10, 1083547 (2023).
Paapstel, K. et al. Inverse relations of serum phosphatidylcholines and lysophosphatidylcholines with vascular damage and heart rate in patients with atherosclerosis. Nutr. Metab. Cardiovasc. Dis. 28(1), 44–52. https://doi.org/10.1016/j.numecd.2017.07.011 (2018).
Ismaeel, A. et al. Altered metabolomic profile in patients with peripheral artery disease. J. Clin. Med. 8(9), 1463. https://doi.org/10.3390/jcm8091463 (2019).
Yao, C. et al. Tryptophan metabolism and ischemic stroke: An intricate balance. Neur. Regen. Res. 21(2), 466–477. https://doi.org/10.4103/NRR.NRR-D-24-00777 (2026).
Wang, S. Q. et al. Assessing the causal relationships between lipid species and stroke by using mendelian randomization. Mol. Neurobiol. 62(6), 7174–7182. https://doi.org/10.1007/s12035-025-04697-9 (2025).
Ismaeel, A., Lavado, R. & Koutakis, P. Metabolomics of peripheral artery disease. Adv. Clin. Chem. 106, 67–89. https://doi.org/10.1016/bs.acc.2021.09.004 (2022).
Lin, S. et al. Neutrophil extracellular traps induced by IL-1beta promote endothelial dysfunction and aggravate limb ischemia. Hypertens Res. 47(6), 1654–1667. https://doi.org/10.1038/s41440-024-01661-3 (2024).
Li, Y. et al. A labeling strategy for the three-dimensional recognition and analysis of microvascular obstruction in ischemic stroke. Theranostics. 13(1), 403–416. https://doi.org/10.7150/thno.76879 (2023).
Longa, E. Z., Weinstein, P. R., Carlson, S. & Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20(1), 84–91 (1989).
Chen, Y., Liao, L., Wang, B. & Wu, Z. Identification and validation of immune and cuproptosis—Related genes for diabetic nephropathy by WGCNA and machine learning. Front. Immunol. 15, 1332279. https://doi.org/10.3389/fimmu.2024.1332279 (2024).
Wang, X. et al. POU class 2 Homeobox associating factor 1, as a hub candidate gene in OP relieves osteoblast apoptosis. Appl. Biochem. Biotechnol. 196(9), 6072–6096. https://doi.org/10.1007/s12010-023-04833-y (2024).
Xu, M. et al. Identification and validation of immune and oxidative stress-related diagnostic markers for diabetic nephropathy by WGCNA and machine learning. Front. Immunol. 14, 1084531. https://doi.org/10.3389/fimmu.2023.1084531 (2023).
Zhang, F. et al. Using machine learning to identify proteomic and metabolomic signatures of stroke in atrial fibrillation. Comput. Biol. Med. 173, 108375. https://doi.org/10.1016/j.compbiomed.2024.108375 (2024).
Wisniewski, K. et al. Computational fluid dynamics; A new diagnostic tool in giant intracerebral aneurysm treatment. Comput. Biol. Med. 181, 109053. https://doi.org/10.1016/j.compbiomed.2024.109053 (2024).
Salinet, A. S. et al. Impaired cerebral autoregulation and neurovascular coupling in middle cerebral artery stroke: Influence of severity. J. Cereb. Blood Flow Metab. 39(11), 2277–2285. https://doi.org/10.1177/0271678X18794835 (2019).
Xu, M. Y. et al. Urolithin A promotes atherosclerotic plaque stability by limiting inflammation and hypercholesteremia in Apolipoprotein E-deficient mice. Acta. Pharmacol. Sin. 45(11), 2277–2289. https://doi.org/10.1038/s41401-024-01317-5 (2024).
Villa-Roel, N. et al. Hypoxia inducible factor 1alpha inhibitor PX-478 reduces atherosclerosis in mice. Atherosclerosis 344, 20–30. https://doi.org/10.1016/j.atherosclerosis.2022.01.002 (2022).
Yang, J. et al. Ertugliflozin attenuates atherosclerosis in nondiabetic ApoE(-/-) mice by upregulating ABCA1 and LDLR via the PPARgamma/LXRalpha pathway. Biochim. Biophys. Acta. Mol. Basis Dis. 1871(7), 167927. https://doi.org/10.1016/j.bbadis.2025.167927 (2025).
Webb, A. J. S., Paolucci, M., Mazzucco, S., Li, L. & Rothwell, P. M. Oxford vascular study phenotyped C. Confounding of cerebral blood flow velocity by blood pressure during breath holding or hyperventilation in transient ischemic attack or stroke. Stroke 51(2), 468–474. https://doi.org/10.1161/STROKEAHA.119.027829 (2020).
Chuang, S. Y. et al. Blood pressure, carotid flow pulsatility, and the risk of stroke: A community-based study. Stroke 47(9), 2262–2268. https://doi.org/10.1161/STROKEAHA.116.013207 (2016).
Hartman, E. M. J. et al. Wall shear stress-related plaque growth of lipid-rich plaques in human coronary arteries: An near-infrared spectroscopy and optical coherence tomography study. Cardiovasc. Res. 119(4), 1021–1029. https://doi.org/10.1093/cvr/cvac178 (2023).
Woo, H. G. et al. Wall shear stress associated with stroke occurrence and mechanisms in middle cerebral artery atherosclerosis. J. Stroke. 25(1), 132–140. https://doi.org/10.5853/jos.2022.02754 (2023).
Sui, B. et al. Hemodynamics parameters distribution of upstream, stenosis center, and downstream sides of plaques in carotid artery with different stenosis: A MRI and CFD study. Acta. Radiol. 56(3), 347–354. https://doi.org/10.1177/0284185114526713 (2015).
Sandeep, S. & Shine, S. R. Effect of stenosis and dilatation on the hemodynamics parameters associated with left coronary artery. Comput. Method. Progr. Biomed. 204, 106052. https://doi.org/10.1016/j.cmpb.2021.106052 (2021).
Jia, Q. et al. Combination of magnetic resonance angiography and computational fluid dynamics may predict the risk of stroke in patients with asymptomatic carotid plaques. Med. Sci. Monit. 23, 479–488. https://doi.org/10.12659/msm.902995 (2017).
Sarkar, P., Kumar, A., Behera, P. S. & Thirumurugan, K. Phytotherapeutic targeting of the mitochondria in neurodegenerative disorders. Adv. Protein Chem. Struct. Biol. 136, 415–455. https://doi.org/10.1016/bs.apcsb.2023.02.013 (2023).
Zheng, M., Wang, W., Liu, J., Zhang, X. & Zhang, R. Lipid metabolism in cancer cells. Adv. Exp. Med. Biol. 1316, 49–69. https://doi.org/10.1007/978-981-33-6785-2_4 (2021).
Rangholia, N., Leisner, T. M. & Holly, S. P. Bioactive ether lipids: Primordial modulators of cellular signaling. Metabolites 11(1), 41. https://doi.org/10.3390/metabo11010041 (2021).
Tang, H. et al. Glycerophospholipid and sphingosine- 1-phosphate metabolism in cardiovascular disease: Mechanisms and therapeutic potential. J. Cardiovasc. Transl. Res. https://doi.org/10.1007/s12265-025-10620-3 (2025).
Lacolley, P., Regnault, V. & Laurent, S. Mechanisms of arterial stiffening: From Mechanotransduction to epigenetics. Arterioscler Thromb. Vasc. Biol. 40(5), 1055–1062. https://doi.org/10.1161/ATVBAHA.119.313129 (2020).
Du, L. D. et al. MMP-9 inhibitor SB-3CT improves neurological outcomes in ischemic stroke mice by modulation of astrocytic lipid metabolism. Acta. Pharmacol. Sin. 46(8), 2120–2135. https://doi.org/10.1038/s41401-025-01505-x (2025).
De Winter, J., Beijer, D., De Ridder, W., Synofzik, M., Zuchner, S. L., consortium P, et al. PCYT2 mutations disrupting etherlipid biosynthesis: phenotypes converging on the CDP-ethanolamine pathway. Brain. 2021;144(2):e17. Epub 2020/11/25. https://doi.org/10.1093/brain/awaa389. PubMed PMID: 33230519.
Wei, Z. et al. p75NTR enhances cognitive dysfunction in a mouse Alzheimer’s disease model by inhibiting microRNA-210–3p-mediated PCYT2 through activation of NF-kappaB. Int. J. Biol. Macromol. 225, 404–415. https://doi.org/10.1016/j.ijbiomac.2022.11.078 (2023).
van Ijzendoorn SC, Agnetti J, Gassama-Diagne A. 2020 Mechanisms behind the polarized distribution of lipids in epithelial cells. Biochimica et Biophysica Acta (BBA) Biomembranes. 1862 (2): 183145.
Hirata, T., Yamamoto, K., Ikeda, K. & Arita, M. Functional lipidomics of vascular endothelial cells in response to laminar shear stress. FASEB J. 35(2), e21301 (2021).
Knuplez, E. & Marsche, G. An updated review of pro-and anti-inflammatory properties of plasma lysophosphatidylcholines in the vascular system. Int. J. Mol. Sci. 21(12), 4501 (2020).
Parnova, R. Critical role of endothelial lysophosphatidylcholine transporter Mfsd2a in maintaining blood–brain barrier integrity and delivering omega 3 PUFA to the brain. J. Evol. Biochem. Physiol. 58(3), 742–754 (2022).
Zhang, L. et al. Vascular lipidomics analysis reveales increased levels of phosphocholine and lysophosphocholine in atherosclerotic mice. Nutr. Metab. 20(1), 1 (2023).
Funding
This work was supported by the National Natural Science Foundation of China (No.81874281 to Hong Guo).
Author information
Authors and Affiliations
Contributions
F. X. and Y. C.: Formal Analysis, Methodology, Writing—original draft, review and editing, Software. S. Y.: Methodology. Y. F.: Data Curation, Formal Analysis. B. W.: Data Curation. B.C.: Validation. H.G.: Funding Acquisition, Project administration, Writing—original draft, review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All animal procedures were approved by the Institutional Animal Care and Use Committee of The First Affiliated Hospital of Harbin Medical University, Harbin, China, and performed in accordance with the Animal Research: Reporting of in vivo Experiments (ARRIVE) guidelines and institutional ethical standards. (Approval No.: 2024046).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xiang, F., Cui, Y., Yang, S. et al. Chronic hindlimb ischemia exacerbates ischemic stroke by disrupting cerebral hemodynamics and glycerophospholipid metabolism in mice. Sci Rep (2026). https://doi.org/10.1038/s41598-025-34372-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-34372-x