Abstract
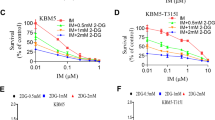
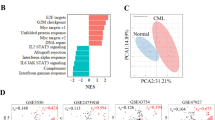
Chronic myeloid leukemia (CML) is an oncogenic hematologic disorder defined by the BCR-ABL1 fusion gene, which initiates pathological proliferation of myeloid lineage cells. While tyrosine kinase inhibitors (TKIs) have substantially improved clinical outcomes, therapeutic resistance associated with the T315I mutation continues to present a significant obstacle. This research evaluates the efficacy of caffeic acid phenethyl ester (CAPE), a bioactive natural product, in countering TKI resistance mediated by this specific genetic alteration. The investigation employed a comprehensive methodology including MTT assay, apoptotic analysis through flow cytometry, proteomic profiling, and bioinformatic interrogation to characterize CAPE’s effects on both wild-type and T315I-mutant CML cellular models. MTT assay indicated that CAPE exhibited potent anti-metabolic activity against CML cells, promoting apoptosis in a dose-dependent manner. Proteomic analysis identified a marked effect of CAPE on the oxidative phosphorylation(OXPHOS) pathway, particularly through the inhibition of mitochondrial complex I(MCI) activity. This inhibition may disrupt cellular energy metabolism, potentially reducing ATP production and heightening susceptibility to cell death. Our findings indicate that CAPE could serve as an adjunctive therapy for CML against drug resistance caused by the T315I mutation through a mechanism that does not directly inhibit BCR-ABL1. This study underscores the promise of targeting mitochondrial metabolism as a novel approach for overcoming therapeutic resistance in CML.
Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Faderl, S. et al. The biology of chronic myeloid leukemia. N. Engl. J. Med. 341, 164–172. https://doi.org/10.1056/nejm199907153410306 (1999).
Senapati, J., Jabbour, E., Kantarjian, H. & Short, N. J. Pathogenesis and management of accelerated and blast phases of chronic myeloid leukemia. Leukemia 37, 5–17, https://doi.org/10.1038/s41375-022-01736-5(2023).
Haddad, F. G. & Short, N. J. Treatment discontinuation in chronic myeloid leukemia: When, how, and why? Am. J. Hematol. 98, 1670–1672. https://doi.org/10.1002/ajh.27100 (2023).
Su, J. et al. Screening and activity evaluation of novel BCR-ABL/T315I tyrosine kinase inhibitors. Curr. Med. Chem. 31, 2872–2894. https://doi.org/10.2174/0929867330666230519105900 (2024).
Verhagen, N. E., Koenderink, J. B., Blijlevens, N. M., Janssen, J. J. & Russel, F. G. Transporter-mediated cellular distribution of tyrosine kinase inhibitors as a potential resistance mechanism in chronic myeloid leukemia. Pharmaceutics 15, 2535. https://doi.org/10.3390/pharmaceutics15112535 (2023).
Soverini, S. et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: recommendations from an expert panel on behalf of European LeukemiaNet. Blood J. Am. Soc. Hematol. 118, 1208–1215. https://doi.org/10.1182/blood-2010-12-326405 (2011).
Lai, H. R., Wu, Q. M., Yang, Y. Z. & Li, J. Recent advance of newly therapy for chronic myeloid leukemia with BCR-ABL T315I mutation–review. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 31, 1579–1583. https://doi.org/10.19746/j.cnki.issn.1009-2137.2023.05.052 (2023).
Minciacchi, V. R., Kumar, R. & Krause, D. S. Chronic myeloid leukemia: a model disease of the past, present and future. Cells 10, 117. https://doi.org/10.1590/s1679-45082011rb2022 (2021).
Kantarjian, H. M. et al. Ponatinib-review of historical development, current status, and future research. Am. J. Hematol. 99, 1576–1585. https://doi.org/10.1002/ajh.27355 (2024).
Zhou, L. Y. Research advance of BCR-ABL mutation and the efficacy of second and third generation TKI in chronic myeloid leukemia–review. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 31, 585–588. https://doi.org/10.19746/j.cnki.issn.1009-2137.2023.02.040 (2023).
Sicuranza, A., Cavalleri, A. & Bernardi, S. The biology of chronic myeloid leukemia: an overview of the new insights and biomarkers. Front. Oncol. 15 https://doi.org/10.3389/fonc.2025.1546813 (2025).
Lv, L., Cui, H., Ma, Z., Liu, X. & Yang, L. Recent progresses in the Pharmacological activities of caffeic acid phenethyl ester. Naunyn-Schmiedeberg’s Archives Pharmacology 394, 1327–1339, https://doi.org/10.1007/s00210-021-02054-w (2021 ).
Chang, K. S. et al. The antitumor effect of caffeic acid phenethyl ester by downregulating mucosa-associated lymphoid tissue 1 via AR/p53/NF-κB signaling in prostate carcinoma cells. Cancers 14, 274. https://doi.org/10.3390/cancers14020274 (2022).
Liang, Y. et al. Caffeic acid phenethyl ester suppressed growth and metastasis of nasopharyngeal carcinoma cells by inactivating the NF-κB pathway. Drug. Des. Devel. Ther. 1335–1345. https://doi.org/10.2147/DDDT.S199182 (2019).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. https://doi.org/10.1093/nar/28.1.27 (2000).
Kanehisa, M., Sato, Y., Kawashima, M., Furumichi, M. & Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 44 https://doi.org/10.1093/nar/gkv1070 (2016). D457-D462.
Li, N. et al. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. Biol. Chem. 278, 8516–8525. https://doi.org/10.1074/jbc.M210432200 (2003).
Murtaza, G. et al. Caffeic acid phenethyl ester and therapeutic potentials. BioMed research international 145342, (2014). https://doi.org/10.1155/2014/145342 (2014).
Cheng, C. C. et al. Caffeic acid phenethyl ester rescues pulmonary arterial hypertension through the Inhibition of AKT/ERK-dependent PDGF/HIF-1α in vitro and in vivo. Int. J. Mol. Sci. 20, 1468. https://doi.org/10.3390/ijms20061468 (2019).
Olgierd, B., Kamila, Ż., Anna, B. & Emilia, M. The pluripotent activities of caffeic acid phenethyl ester. Molecules 26, 1335. https://doi.org/10.3390/molecules26051335 (2021).
Bjørklund, G. et al. Caffeic acid phenethyl ester: A potential therapeutic cancer agent? Curr. Med. Chem. 31, 6760–6774. https://doi.org/10.2174/0109298673252993230921073502 (2024).
Kabała-Dzik, A. et al. Comparison of two components of propolis: caffeic acid (CA) and caffeic acid phenethyl ester (CAPE) induce apoptosis and cell cycle arrest of breast cancer cells MDA-MB-231. Molecules 22, 1554. https://doi.org/10.3390/molecules22091554 (2017).
Akyol, S. et al. In vivo and in vitro antıneoplastic actions of caffeic acid phenethyl ester (CAPE): therapeutic perspectives. Nutr. Cancer. 65, 515–526. https://doi.org/10.1080/01635581.2013.776693 (2013).
Lin, H. P. et al. Caffeic acid phenethyl ester induced cell cycle arrest and growth inhibition in androgen-independent prostate cancer cells via regulation of Skp2, p53, p21Cip1 and p27Kip1. Oncotarget 6, 6684, https://doi.org/10.18632/oncotarget.3246 (2015).
Lin HuiPing, L. H. et al. Caffeic acid phenethyl ester as a potential treatment for advanced prostate cancer targeting Akt signaling. https://doi.org/10.3390/ijms14035264 (2013).
Motawi, T. K., Abdelazim, S. A., Darwish, H. A., Elbaz, E. M. & Shouman, S. A. Could caffeic acid phenethyl ester expand the antitumor effect of Tamoxifen in breast carcinoma? Nutr. Cancer. 68, 435–445. https://doi.org/10.1080/01635581.2016.1153669 (2016).
Kulkarni, N. P., Vaidya, B., Narula, A. S. & Sharma, S. S. Neuroprotective potential of caffeic acid phenethyl ester (CAPE) in CNS disorders: mechanistic and therapeutic insights. Curr. Neuropharmacol. 19, 1401–1415. https://doi.org/10.2174/1570159X19666210608165509 (2021).
Pandey, P., Khan, F., Upadhyay, T. K. & Giri, P. P. Therapeutic efficacy of caffeic acid phenethyl ester in cancer therapy: an updated review. Chem. Biol. Drug Des. 102, 201–216. https://doi.org/10.1111/cbdd.14233 (2023).
Guan, C., Zhou, X., Li, H., Ma, X. & Zhuang, J. NF-κB inhibitors gifted by nature: the anticancer promise of polyphenol compounds. Biomed. Pharmacother. 156, 113951. https://doi.org/10.1016/j.biopha.2022.113951 (2022).
Heinz, S. et al. Mechanistic investigations of the mitochondrial complex I inhibitor rotenone in the context of Pharmacological and safety evaluation. Sci. Rep. 7, 1–13. https://doi.org/10.1038/srep45465 (2017). www.sci-hub.se/
Bushweller, J. H. Targeting transcription factors in cancer—from undruggable to reality. Nat. Rev. Cancer. 19, 611–624. https://doi.org/10.1038/s41568-019-0196-7 (2019).
Bosc, C. et al. Mitochondrial inhibitors circumvent adaptive resistance to venetoclax and cytarabine combination therapy in acute myeloid leukemia. Nat. Cancer. 2, 1204–1223. https://doi.org/10.1038/s43018-021-00264-y (2021).
Pontis, F. et al. Circulating extracellular vesicles from individuals at high-risk of lung cancer induce pro-tumorigenic conversion of stromal cells through transfer of miR-126 and miR-320. J. Experimental Clin. Cancer Res. 40 https://doi.org/10.1186/s13046-021-02040-3 (2021).
Nolfi-Donegan, D., Braganza, A. & Shiva, S. Mitochondrial electron transport chain: oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 37, 101674. https://doi.org/10.1016/j.redox.2020.101674 (2020).
Martínez-Reyes, I. & Chandel, N. S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 11, 102. https://doi.org/10.1038/s41467-019-13668-3 (2020).
Kalpage, H. A. et al. Tissue-specific regulation of cytochrome c by post-translational modifications: respiration, the mitochondrial membrane potential, ROS, and apoptosis. FASEB J. 33, 1540. https://doi.org/10.1096/fj.201801417R (2018).
Li, Y., Zeng, P., Xiao, J., Huang, P. & Liu, P. Modulation of energy metabolism to overcome drug resistance in chronic myeloid leukemia cells through induction of autophagy. Cell. Death Discovery. 8, 212. https://doi.org/10.1038/s41420-022-00991-w (2022).
Feng, L. et al. BCR-ABL triggers a glucose-dependent survival program during leukemogenesis through the suppression of TXNIP. Cell Death Dis. 14, 287. https://doi.org/10.1038/s41419-023-05811-2 (2023).
Zhang, H. et al. Mechanistic insights into co-administration of allosteric and orthosteric drugs to overcome drug-resistance in T315I BCR-ABL1. Front. Pharmacol. 13, 862504. https://doi.org/10.3389/fphar.2022.862504 (2022).
Burke, A. C., Swords, R. T., Kelly, K. & Giles, F. J. Current status of agents active against the T315I chronic myeloid leukemia phenotype. Expert Opin. Emerg. Drugs. 16, 85–103. https://doi.org/10.1517/14728214.2011.531698 (2011).
Gao, C. et al. I13 overrides resistance mediated by the T315I mutation in chronic myeloid leukemia by direct BCR-ABL Inhibition. Front. Pharmacol. 14, 1183052. https://doi.org/10.3389/fphar.2023.1183052 (2023).
Alton, E. W. et al. A randomised, double-blind, placebo-controlled trial of repeated nebulisation of non-viral cystic fibrosis transmembrane conductance regulator (CFTR) gene therapy in patients with cystic fibrosis. Efficacy Mechanism Evaluation. 3, 1–210. https://doi.org/10.3310/eme03050 (2016).
Sánchez-Sánchez, B. et al. NADPH oxidases as therapeutic targets in chronic myelogenous leukemia. Clin. Cancer Res. 20, 4014–4025. https://doi.org/10.1158/1078-0432.CCR-13-3044 (2014).
Allegra, A. et al. Oxidative stress and chronic myeloid leukemia: a balance between ROS-mediated pro-and anti-apoptotic effects of tyrosine kinase inhibitors. Antioxidants 13, 461. https://doi.org/10.3390/antiox13040461 (2024).
Cavalleri, A. et al. Different in vitro models of chronic myeloid leukemia show different characteristics: biological replicates are not biologically equivalent. Cell. Biol. Int. 49, 570–586. https://doi.org/10.1002/cbin.70007 (2025).
Mutti, S. et al. Assessment of chronic myeloid leukaemia in vitro models variability: insights into extracellular vesicles. J. Cell. Mol. Med. 29 https://doi.org/10.1111/jcmm.70901 (2025).
Acknowledgements
The authors gratefully acknowledge the Dink Laboratory at the Jinan University School of Pharmacy for generously providing the cell lines utilized in this investigation.
Funding
This work was supported by funding from the Guangdong Province Administration of Chinese Medicine Research Project (Grant No. 20221330) and the Futian Healthcare Research Project (Grant Nos. FTWS2022027, FTWS2022059, FTWS2023071).
Author information
Authors and Affiliations
Contributions
Conceptualization, Meng Li and Dongxue Liu; Methodology, Meng Li and Zhiwei Zhang; Software, Zhiwei Zhang; Validation, Meng Li, Dongxue Liu, and Biqian Fu; Formal Analysis, Meng Li and Zhihua Peng; Investigation, Meng Li, Dongxue Liu, Han Han and Ying Zhang; Resources, Han Han, Ying Zhang and Ruihua Xiong; Data Curation, Meng Li and Biqian Fu; Writing – Original Draft Preparation, Meng Li; Writing – Review & Editing, Meng Li, Dongxue Liu, Zhiwei Zhang, Biqian Fu, Ruihua Xiong, and Yanli Fu; Visualization, Zhiwei Zhang; Supervision, Yuhe Lei; Project Administration, Yanli Fu; Funding Acquisition, Yanli Fu.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Funding
This research was funded by the Shenzhen Association of Chinese Medicine (Grant No. 2024140 F) and the Futian Healthcare Research Project (Grant Nos. FTWS2022027, FTWS2022059, FTWS2023071).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, M., Liu, D., Zhang, Z. et al. Caffeic acid phenethyl ester induced apoptosis in chronic myeloid leukemia cells by inhibiting mitochondrial complex I. Sci Rep (2026). https://doi.org/10.1038/s41598-025-34553-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-34553-8