Abstract
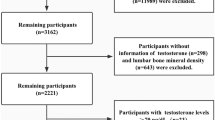
While metabolomics offers insights into metabolic diseases, plasma metabolites in thalassemia patients with low bone mineral density (BMD) have not been explored. This cross-sectional study investigated plasma metabolite alterations in thalassemia patients with low BMD compared to those with normal BMD and healthy controls at Chiang Mai University’s Hematology Clinic. Eighty thalassemia patients and 40 age- and sex-matched controls were enrolled. BMD was measured at two skeletal sites, the lumbar spine (L1–L4) and hip, using dual-energy X-ray absorptiometry. Targeted plasma metabolomics and bone turnover markers (β-CTX-I, total PINP) were assessed. Low BMD was defined as a Z-score ≤ − 2 at any site, and its prevalence among thalassemia patients was 47.5%. Compared to those with normal BMD, thalassemia patients with low BMD showed elevated glutamate, arachidonic acid, and medium- to long-chain acylcarnitines, but reduced glutamine levels. Phosphatidylinositol and lysophosphatidylinositol were also increased. β-CTX-I and total PINP levels did not differ between thalassemia groups. A predictive model using key metabolites (glutamine, arachidonic acid, asparagine, lysoPI (18:0), myristoylcarnitine) showed fair discriminatory ability for low BMD (AUC 0.762, p = 0.007). Thalassemia with low BMD is associated with glutamate–glutamine metabolism disruptions, impaired fatty acid oxidation, and elevated phosphatidylinositol levels.
Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available due to privacy or ethical restrictions.
References
Cappellini, M-D., Cohen, A., Porter, J., Taher, A. & Viprakasit, V. Guidelines for the management of transfusion dependent thalassaemia (TDT). Nicosia (CY): Thalassaemia International Federation (2014). https://www.ncbi.nlm.nih.gov/books/NBK269382/?report=classic
Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 94(6), 646–650 (1993).
Gordon, C. M. et al. Dual energy X-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD pediatric official positions. J. Clin. Densitom. 11 (1), 43–58 (2008).
Sutipornpalangkul, W., Janechetsadatham, Y., Siritanaratkul, N. & Harnroongroj, T. Prevalence of fractures among Thais with thalassaemia syndromes. Singap. Med. J. 51 (10), 817–821 (2010).
Zhang, X., Li, Q., Xu, Z. & Dou, J. Mass spectrometry-based metabolomics in health and medical science: a systematic review. RSC Adv. 10 (6), 3092–3104 (2020).
Zhao, Q. et al. Metabolomic profiles associated with bone mineral density in US Caucasian women. Nutr. Metab. (Lond). 15, 57 (2018).
Miyamoto, T. et al. A serum metabolomics-based profile in low bone mineral density postmenopausal women. Bone 95, 1–4 (2017).
Wang, J. et al. Discovery of potential biomarkers for osteoporosis using LC-MS/MS metabolomic methods. Osteoporos. Int. 30 (7), 1491–1499 (2019).
Musharraf, S. G. et al. beta-Thalassemia patients revealed a significant change of untargeted metabolites in comparison to healthy individuals. Sci. Rep. 7, 42249 (2017).
Wong, P., Fuller, P. J., Gillespie, M. T. & Milat, F. Bone disease in thalassemia: A molecular and clinical overview. Endocr. Rev. 37 (4), 320–346 (2016).
Lewiecki, E. M. et al. International society for clinical densitometry 2007 adult and pediatric official positions. Bone 43 (6), 1115–1121 (2008).
Thonusin, C. et al. Blood metabolomes as non-invasive biomarkers and targets of metabolic interventions for doxorubicin and trastuzumab-induced cardiotoxicity. Arch. Toxicol. 97 (2), 603–618 (2023).
Thonusin, C. et al. Evaluation of intensity drift correction strategies using MetaboDrift, a normalization tool for multi-batch metabolomics data. J. Chromatogr. A. 1523, 265–274 (2017).
Mahachoklertwattana, P. et al. Bone mineral density, biochemical and hormonal profiles in suboptimally treated children and adolescents with beta-thalassaemia disease. Clin. Endocrinol. (Oxf). 58 (3), 273–279 (2003).
Cochran, W. G. Sampling Techniques, 3d edn., xvi (Wiley, 1977).
Pang, Z. et al. MetaboAnalyst 6.0: towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 52 (W1), W398–W406 (2024).
Chen, X. et al. Amniotic fluid metabolomic and lipidomic alterations associated with hemoglobin bart’s diseases. Metabolomics 17 (9), 82 (2021).
Monni, G. et al. Metabolomic investigation of beta-Thalassemia in chorionic villi samples. J. Clin. Med. ;8(6). (2019).
Iqbal, A. et al. Hydroxyurea treated beta-Thalassemia children demonstrate a shift in metabolism towards healthy pattern. Sci. Rep. 8 (1), 15152 (2018).
Tzounakas, V. L. et al. Beta thalassemia minor is a beneficial determinant of red blood cell storage lesion. Haematologica 107 (1), 112–125 (2022).
Hortle, E. et al. Adenosine monophosphate deaminase 3 activation shortens erythrocyte half-life and provides malaria resistance in mice. Blood 128 (9), 1290–1301 (2016).
Rejnmark, L., Vestergaard, P., Brot, C. & Mosekilde, L. Parathyroid response to vitamin D insufficiency: relations to bone, body composition and to lifestyle characteristics. Clin. Endocrinol. (Oxf). 69 (1), 29–35 (2008).
Rodbro, L. L., Bislev, L. S., Sikjaer, T. & Rejnmark, L. Bone metabolism, density, and geometry in postmenopausal women with vitamin D insufficiency: a cross-sectional comparison of the effects of elevated parathyroid levels. Osteoporos. Int. 29 (10), 2211–2218 (2018).
Tsartsalis, A. N. et al. Bone metabolism markers in thalassemia major-induced osteoporosis: results from a cross-sectional observational study. Curr. Mol. Med. 19 (5), 335–341 (2019).
Celik, T., Sangun, O., Unal, S., Balci, A. & Motor, S. Assessment of biochemical bone markers of osteoporosis in children with thalassemia major. Ital. J. Pediatr. 48 (1), 105 (2022).
Das, L. et al. Bone turnover, areal BMD, and bone microarchitecture by second-generation high-resolution peripheral quantitative computed tomography in transfusion-dependent thalassemia. JBMR Plus. 8 (11), ziae117 (2024).
Baldini, M. et al. Thalassemic osteopathy: a new marker of bone deposition. Blood Cells Mol. Dis. 52 (2–3), 91–94 (2014).
Abdulrazzaq, Y. M., Ibrahim, A., Al-Khayat, A. I. & Dawson, K. Beta-thalassemia major and its effect on amino acid metabolism and growth in patients in the united Arab Emirates. Clin. Chim. Acta. 352 (1–2), 183–190 (2005).
Lyu, J. et al. A glutamine metabolic switch supports erythropoiesis. Science 386 (6723), eadh9215 (2024).
Kalpravidh, R. W. et al. Glutathione redox system in beta -thalassemia/Hb E patients. ScientificWorldJournal 2013, 543973 (2013).
Devignes, C. S., Carmeliet, G. & Stegen, S. Amino acid metabolism in skeletal cells. Bone Rep. 17, 101620 (2022).
Stegen, S. et al. Glutamine metabolism in osteoprogenitors is required for bone mass accrual and PTH-induced bone anabolism in male mice. J. Bone Min. Res. 36 (3), 604–616 (2021).
Yu, Y. et al. Glutamine metabolism regulates proliferation and lineage allocation in skeletal stem cells. Cell. Metab. 29 (4), 966–78e4 (2019).
Wauquier, F., Leotoing, L., Coxam, V., Guicheux, J. & Wittrant, Y. Oxidative stress in bone remodelling and disease. Trends Mol. Med. 15 (10), 468–477 (2009).
Domazetovic, V., Marcucci, G., Iantomasi, T., Brandi, M. L. & Vincenzini, M. T. Oxidative stress in bone remodeling: role of antioxidants. Clin. Cases Min. Bone Metab. 14 (2), 209–216 (2017).
Baek, K. H. et al. Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif Tissue Int. 87 (3), 226–235 (2010).
Dzubanova, M. et al. Glutamine: A novel player in maintaining skeletal strength and body fitness in obese mice. Clin. Nutr. 54, 162–176 (2025).
Bertolo, R. F. & Burrin, D. G. Comparative aspects of tissue glutamine and proline metabolism. J. Nutr. 138 (10), 2032S–9S (2008).
Gelse, K., Poschl, E. & Aigner, T. Collagens–structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 55 (12), 1531–1546 (2003).
Bahar, A., Kashi, Z., Sohrab, M., Kosaryan, M. & Janbabai, G. Relationship between beta-globin gene carrier state and insulin resistance. J. Diabetes Metab. Disord. 11 (1), 22 (2012).
Würtz, P. et al. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care. 36 (3), 648–655 (2013).
Lynch, C. J. & Adams, S. H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 10 (12), 723–736 (2014).
Menni, C. et al. Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes 62 (12), 4270–4276 (2013).
Conte, C., Epstein, S. & Napoli, N. Insulin resistance and bone: a biological partnership. Acta Diabetol. 55 (4), 305–314 (2018).
Epstein, S., Defeudis, G., Manfrini, S., Napoli, N. & Pozzilli, P. Diabetes and disordered bone metabolism (diabetic osteodystrophy): time for recognition. Osteoporos. Int. 27 (6), 1931–1951 (2016).
Leslie, W. D., Rubin, M. R., Schwartz, A. V. & Kanis, J. A. Type 2 diabetes and bone. J. Bone Min. Res. 27 (11), 2231–2237 (2012).
Gilli, G., Moiraghi Ruggenini, A., Nani, E., Bottura, G. & Mastretta, L. Study of the fatty acid components of the triglyceride fraction of the blood in normal and thalassemic subjects, using gas chromatography. Arch. Sci. Med. (Torino). 134 (3), 293–300 (1977).
Zhan, Q. et al. The opposite effects of Antarctic Krill oil and arachidonic acid-rich oil on bone resorption in ovariectomized mice. Food Funct. 11 (8), 7048–7060 (2020).
Casado-Diaz, A., Santiago-Mora, R., Dorado, G. & Quesada-Gomez, J. M. The omega-6 arachidonic fatty acid, but not the omega-3 fatty acids, inhibits osteoblastogenesis and induces adipogenesis of human mesenchymal stem cells: potential implication in osteoporosis. Osteoporos. Int. 24 (5), 1647–1661 (2013).
Zheng, D. M. et al. Medium and long-chain acylcarnitine’s relation to lipid metabolism as potential predictors for diabetic cardiomyopathy: a metabolomic study. Lipids Health Dis. 20 (1), 151 (2021).
Schooneman, M. G., Vaz, F. M., Houten, S. M. & Soeters, M. R. Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes 62 (1), 1–8 (2013).
Paapstel, K. et al. Metabolomic profiles of lipid metabolism, arterial stiffness and hemodynamics in male coronary artery disease patients. IJC Metab. Endocr. 11, 13–18 (2016).
Chen, H. et al. Comparative proteomics reveals that fatty acid metabolism is involved in myocardial adaptation to chronic hypoxic injury. PLoS One. 19 (6), e0305571 (2024).
Huang, Z. et al. CPT1A-mediated fatty acid oxidation promotes precursor osteoclast fusion in rheumatoid arthritis. Front. Immunol. 13, 838664 (2022).
Aleidi, S. M. et al. Lipidomics profiling of patients with low bone mineral density (LBMD). Int. J. Mol. Sci. 23, 19 (2022).
Ke, J. Y. et al. Iron overload induces apoptosis of murine preosteoblast cells via ROS and inhibition of AKT pathway. Oral Dis. 23 (6), 784–794 (2017).
Wang, L. et al. Deletion of ferroportin in murine myeloid cells increases iron accumulation and stimulates osteoclastogenesis in vitro and in vivo. J. Biol. Chem. 293 (24), 9248–9264 (2018).
Zhang, X. et al. Metabolomics insights into osteoporosis through association with bone mineral density. J. Bone Min. Res. (2021).
Wang, J., Wang, Y., Zeng, Y. & Huang, D. Feature selection approaches identify potential plasma metabolites in postmenopausal osteoporosis patients. Metabolomics 18 (11), 86 (2022).
Aleidi, S. M. et al. A distinctive human metabolomics alteration associated with osteopenic and osteoporotic patients. Metabolites ;11(9). (2021).
Mei, Z. et al. Association between the metabolome and bone mineral density in a Chinese population. EBioMedicine 62, 103111 (2020).
Piriyakhuntorn, P. et al. Melatonin supplementation alleviates bone mineral density decline and circulating oxidative stress in iron-overloaded thalassemia patients. J. Pineal Res. 77 (3), e70055 (2025).
Funding
This research was supported by a research grant from OPS MHESI, TSRI, and Chiang Mai University (RGNS 64-072: P.P.); a grant from the Thai Society of Hematology (P.P.); the Mid-Career Research Grant from the National Research Council of Thailand (C.T.); the Distinguished Research Professor Grant from the National Research Council of Thailand (N42A660301: S.C.C); and a Chiang Mai University Center of Excellence Award (N.C.)
Author information
Authors and Affiliations
Contributions
P.P.: Conceptualization, methodology, formal analysis, investigation, writing-original draft, funding acquisition; S.C.: Conceptualization, writing-review and editing, supervision; A.T.: Conceptualization, writing-review and editing; P.N.: Methodology, investigation; C.T.: Methodology, formal analysis, writing-review and editing; W.N.: Investigation; T.K.: Investigation; N.C.: Writing-review & editing; All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work, the author(s) used ChatGPT 4o in order to improve readability and language. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the published article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Piriyakhuntorn, P., Tantiworawit, A., Niprapan, P. et al. Prevalence of low bone mineral density and associated plasma metabolite alterations in thalassemia. Sci Rep (2026). https://doi.org/10.1038/s41598-025-34667-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-34667-z