Abstract
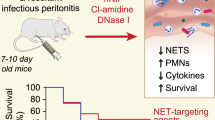
There are no specific treatments for Sepsis-associated acute kidney injury (AKI). We previously reported that Il-17a-knockout mice had dramatically improved survival after cecal ligation and puncture (CLP). Neutrophil extracellular traps (NETs) induce IL-17A, which causes harm in some diseases, but this pathway is poorly understood in sepsis. We found that knockout of Pad4 (Peptidyl Arginine Deiminase 4), an enzyme essential for NET formation, improved survival and AKI, and suppressed neutrophil infiltration into remote organs, involving a peritoneal IL-17A/distant organ CXCL-1/CXCL-2 pathway after CLP. NETs were detected in the peritoneal cavity, and not in plasma or distant organs. Adoptive transfer of peritoneal WT neutrophils restored the IL-17A/CXCL-1/CXCL-2 pathway in Pad4KO mice, leading to neutrophil infiltration and damage to remote organs. These results revealed a pathway from peritoneal NET formation to remote organ injury/inflammation via production of IL-17A at the infectious site and distant organ CXCL-1/CXCL-2. While NETs promoted intraperitoneal IL-17A production, we also showed that conversely, peritoneal IL-17A or CXCL-1/CXCL-2 promoted intraperitoneal NET formation after CLP. This peritoneal vicious cycle that includes NET formation, IL-17A, CXCL-1/CXCL-2 that may amplify sepsis-associated organ injury. Breaking this vicious cycle by inhibiting NET formation and/or IL-17A might be a promising therapeutic target for sepsis treatment.
Similar content being viewed by others
Data availability
Underlying data are published on Mendeley Data (Reserved https://doi.org/10.17632/y2tbj7h96t.1).
References
Peerapornratana, S., Manrique-Caballero, C. L., Gómez, H. & Kellum, J. A. Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 96(5), 1083–1099. https://doi.org/10.1016/j.kint.2019.05.026 (2019).
Bagshaw, S. M. et al. Acute kidney injury in septic shock: Clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med. https://doi.org/10.1007/s00134-008-1367-2 (2009).
Poston, J. T. & Koyner, J. L. Sepsis associated acute kidney injury. BMJ 364(9), k4891–k4891. https://doi.org/10.1136/bmj.k4891 (2019).
Bagshaw, S. M. et al. Septic acute kidney injury in critically ill patients: Clinical characteristics and outcomes. Clin. J. Am. Soc. Nephrol. 2(3), 431–439. https://doi.org/10.2215/CJN.03681106 (2007).
Czermak, B. J. et al. Mechanisms of enhanced lung injury during sepsis. Am. J. Pathol. 154(4), 1057–1065. https://doi.org/10.1016/s0002-9440(10)65358-8 (1999).
Li, B. et al. Crosstalk between lung and extrapulmonary organs in sepsis-related acute lung injury/acute respiratory distress syndrome. Ann. Intensive Care. 15(1), 97. https://doi.org/10.1186/s13613-025-01513-4 (2025).
Xie, B. et al. Gut-derived memory γδ T17 cells exacerbate sepsis-induced acute lung injury in mice. Nat. Commun. 15(1), 6737. https://doi.org/10.1038/s41467-024-51209-9 (2024).
Sun, B. et al. Acute lung injury caused by sepsis: How does it happen?. Front. Med. (Lausanne). 10, 1289194. https://doi.org/10.3389/fmed.2023.1289194 (2023).
Charoensappakit, A. et al. Cell-free DNA as diagnostic and prognostic biomarkers for adult sepsis: A systematic review and meta-analysis. Sci. Rep. 13(1), 19624. https://doi.org/10.1038/s41598-023-46663-2 (2023).
Denning, N. L., Aziz, M., Gurien, S. D. & Wang, P. Damps and nets in sepsis. Front. Immunol. 10(OCT), 1–15. https://doi.org/10.3389/fimmu.2019.02536 (2019).
Li, R. H. L. & Tablin, F. A comparative review of neutrophil extracellular traps in sepsis. Front. Vet. Sci. 5, 291. https://doi.org/10.3389/fvets.2018.00291 (2018).
Takei, H., Araki, A., Watanabe, H., Ichinose, A. & Sendo, F. Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J. Leukoc Biol. 59(2), 229–240. https://doi.org/10.1002/jlb.59.2.229 (1996).
Singh, J. et al. Moonlighting chromatin: When DNA escapes nuclear control. Cell Death Differ. 30(4), 861–875. https://doi.org/10.1038/s41418-023-01124-1 (2023).
Rohrbach, A. S., Slade, D. J., Thompson, P. R. & Mowen, K. A. Activation of PAD4 in NET formation. Front. Immunol. 3(NOV), 1–10. https://doi.org/10.3389/fimmu.2012.00360 (2012).
Wang, Y. et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J. Cell Biol. 184(2), 205–213. https://doi.org/10.1083/jcb.200806072 (2009).
Li, Y. et al. Nuclear envelope rupture and NET formation is driven by PKCα-mediated lamin B disassembly. EMBO Rep. 21(8), e48779. https://doi.org/10.15252/embr.201948779 (2020).
Amulic, B. et al. Cell-cycle proteins control production of neutrophil extracellular traps. Dev Cell. 43(4), 449-462.e5. https://doi.org/10.1016/j.devcel.2017.10.013 (2017).
Li, M., Lyu, X., Liao, J., Werth, V. P. & Liu, M. L. Rho Kinase regulates neutrophil NET formation that is involved in UVB-induced skin inflammation. Theranostics. 12(5), 2133–2149. https://doi.org/10.7150/thno.66457 (2022).
Martinod, K. et al. PAD4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood 125(12), 1948–1956. https://doi.org/10.1182/blood-2014-07-587709 (2015).
McDonald, B., Urrutia, R., Yipp, B. G., Jenne, C. N. & Kubes, P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe. 12(3), 324–333. https://doi.org/10.1016/j.chom.2012.06.011 (2012).
McDonald, B. et al. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood 129(10), 1357–1367. https://doi.org/10.1182/blood-2016-09-741298 (2017).
Tian, Y. et al. Citrullinated histone H3 mediates sepsis-induced lung injury through activating caspase-1 dependent inflammasome pathway. Front. Immunol. 12(December), 4–7. https://doi.org/10.3389/fimmu.2021.761345 (2021).
Biron, B. M. et al. Cl-Amidine prevents histone 3 citrullination and neutrophil extracellular trap formation, and improves survival in a murine sepsis model. J. Innate Immun. 9(1), 22–32. https://doi.org/10.1159/000448808 (2017).
Czaikoski, P. G. et al. Neutrophil extracellular traps induce organ damage during experimental and clinical sepsis. PLoS ONE https://doi.org/10.1371/journal.pone.0148142 (2016).
Naito, Y. et al. IL-17A activated by Toll-like receptor 9 contributes to the development of septic acute kidney injury. Am. J. Physiol. Renal Physiol. 318(1), F238–F247. https://doi.org/10.1152/ajprenal.00313.2019 (2020).
Plitas, G., Burt, B. M., Nguyen, H. M., Bamboat, Z. M. & DeMatteo, R. P. Toll-like receptor 9 inhibition reduces mortality in polymicrobial sepsis. J. Exp. Med. 205(6), 1277–1283. https://doi.org/10.1084/jem.20080162 (2008).
Yasuda, H. et al. Chloroquine and inhibition of Toll-like receptor 9 protect from sepsis-induced acute kidney injury. Am. J. Physiol.-Renal Physiol. 294(5), F1050–F1058. https://doi.org/10.1152/ajprenal.00461.2007 (2008).
Wilson, A. S. et al. Neutrophil extracellular traps and their histones promote Th17 cell differentiation directly via TLR2. Nat. Commun. 13(1), 1–12. https://doi.org/10.1038/s41467-022-28172-4 (2022).
Liu, Y. et al. Myeloid-specific deletion of peptidylarginine deiminase 4 mitigates atherosclerosis. Front. Immunol. https://doi.org/10.3389/fimmu.2018.01680 (2018).
Ge, Y., Huang, M. & Yao, Y. M. Biology of Interleukin-17 and Its pathophysiological significance in sepsis. Front. Immunol. 11(July), 1–13. https://doi.org/10.3389/fimmu.2020.01558 (2020).
Tsuji, N. et al. BAM15 treats mouse sepsis and kidney injury, linking mortality, mitochondrial DNA, tubule damage, and neutrophils. J. Clin. Investig. https://doi.org/10.1172/JCI152401 (2023).
Ocuin, L. M. et al. Neutrophil IL-10 suppresses peritoneal inflammatory monocytes during polymicrobial sepsis. J. Leukoc Biol. 89(3), 423–432. https://doi.org/10.1189/jlb.0810479 (2011).
Li, R. H. L. & Tablin, F. A comparative review of neutrophil extracellular traps in sepsis. Front. Vet. Sci. 5(NOV), 1–11. https://doi.org/10.3389/fvets.2018.00291 (2018).
Ciesielski, O. et al. Citrullination in the pathology of inflammatory and autoimmune disorders: Recent advances and future perspectives. Cell. Mol. Life Sci. 79(2), 1–20. https://doi.org/10.1007/s00018-022-04126-3 (2022).
Li, P. et al. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 207(9), 1853–1862. https://doi.org/10.1084/jem.20100239 (2010).
Li, Y. et al. Citrullinated histone H3: A novel target for the treatment of sepsis. Surgery. 156(2), 229–234. https://doi.org/10.1016/j.surg.2014.04.009 (2014).
Huang, Y. et al. Activated platelets autocrine 5-hydroxytryptophan aggravates sepsis-induced acute lung injury by promoting neutrophils extracellular traps formation. Front. Cell Dev. Biol. 9(January), 1–10. https://doi.org/10.3389/fcell.2021.777989 (2022).
Zhu, Y. et al. Zingerone inhibits the neutrophil extracellular trap formation and protects against sepsis via Nrf2-mediated ROS inhibition. Oxid. Med. Cell. Longev. 2022, 1–16. https://doi.org/10.1155/2022/3990607 (2022).
Luo, L. et al. Proinflammatory role of neutrophil extracellular traps in abdominal sepsis. Am. J. Physiol. Lung Cell. Mol. Physiol. 307(7), L586–L596. https://doi.org/10.1152/ajplung.00365.2013 (2014).
Alsabani, M. et al. Reduction of NETosis by targeting CXCR1/2 reduces thrombosis, lung injury, and mortality in experimental human and murine sepsis. Br. J. Anaesth. 128(2), 283–293. https://doi.org/10.1016/j.bja.2021.10.039 (2022).
Shang, T. et al. Xuebijing injection inhibited neutrophil extracellular traps to reverse lung injury in sepsis mice via reducing Gasdermin D. Front. Pharmacol. 13(November), 1–15. https://doi.org/10.3389/fphar.2022.1054176 (2022).
Ni, Y. et al. Interruption of neutrophil extracellular traps formation dictates host defense and tubular HOXA5 stability to augment efficacy of anti-Fn14 therapy against septic AKI. Theranostics. 11(19), 9431–9451. https://doi.org/10.7150/thno.61902 (2021).
Ito, T. et al. Circulating histone H3 levels are increased in septic mice in a neutrophil-dependent manner: Preclinical evaluation of a novel sandwich ELISA for histone H3. J. Intens. Care 6(1), 1–6. https://doi.org/10.1186/s40560-018-0348-y (2018).
Flierl, M. A. et al. Adverse functions of IL-17A in experimental sepsis. FASEB J. Off. Publ. Federation Am. Soc. Exp. Biol. 22, 2198–2205. https://doi.org/10.1096/fj.07-105221 (2008).
Girbl, T. et al. Distinct compartmentalization of the chemokines CXCL1 and CXCL2 and the atypical receptor ACKR1 determine discrete stages of neutrophil diapedesis. Immunity 49(6), 1062-1076.e6. https://doi.org/10.1016/j.immuni.2018.09.018 (2018).
Luo, C.-J. et al. Knockout of interleukin-17A protects against sepsis-associated acute kidney injury. Ann. Intens. Care 6(1), 56–56. https://doi.org/10.1186/s13613-016-0157-1 (2016).
Tohme, S. et al. Computational analysis supports IL-17A as a central driver of neutrophil extracellular trap-mediated injury in liver ischemia reperfusion. J. Immunol. 202(1), 268–277. https://doi.org/10.4049/jimmunol.1800454 (2019).
Jin, L., Batra, S. & Jeyaseelan, S. Diminished neutrophil extracellular trap (NET) formation is a novel innate immune deficiency induced by acute ethanol exposure in polymicrobial sepsis, which can be rescued by CXCL1. PLoS Pathog. 13(9), 1–24. https://doi.org/10.1371/journal.ppat.1006637 (2017).
Zhang, Y. et al. Interleukin-17-induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J. Exp. Med. https://doi.org/10.1084/jem.20190354 (2020).
Koushik, S. et al. PAD4: Pathophysiology, current therapeutics and future perspective in rheumatoid arthritis. Expert Opin. Ther. Targets. 21(4), 433–447. https://doi.org/10.1080/14728222.2017.1294160 (2017).
Pertiwi, K. R. et al. Extracellular traps derived from macrophages, mast cells, eosinophils and neutrophils are generated in a time-dependent manner during atherothrombosis. J. Pathol. 247(4), 505–512. https://doi.org/10.1002/path.5212 (2019).
Colciaghi, F. & Costanza, M. Unveiling leukocyte extracellular traps in inflammatory responses of the central nervous system. Front Immunol. 13, 915392. https://doi.org/10.3389/fimmu.2022.915392 (2022).
Chang, X. et al. The inhibition of antithrombin by peptidylarginine deiminase 4 may contribute to pathogenesis of rheumatoid arthritis. Rheumatol. (Oxf.) 44(3), 293–298. https://doi.org/10.1093/rheumatology/keh473 (2005).
Pokhrel, S. et al. Complement receptor 3 contributes to the sexual dimorphism in neutrophil killing of staphylococcus aureus. J. Immunol. 205(6), 1593–1600. https://doi.org/10.4049/jimmunol.2000545 (2020).
Gupta, S. et al. Sex differences in neutrophil biology modulate response to type I interferons and immunometabolism. Proc. Natl. Acad. Sci. U. S. A. 117(28), 16481–16491. https://doi.org/10.1073/pnas.2003603117 (2020).
Richter, M., Maier-Begandt, D., Jablonska, J. & Silvestre-Roig, C. Sex differences in neutrophil biology. J. Leukoc. Biol. https://doi.org/10.1093/jleuko/qiaf161 (2025).
Yasuda, H. et al. 17-β-estradiol enhances neutrophil extracellular trap formation by interaction with estrogen membrane receptor. Arch. Biochem. Biophys. 663, 64–70. https://doi.org/10.1016/j.abb.2018.12.028 (2019).
Zhang, P. et al. Estradiol inhibits fMLP-induced neutrophil migration and superoxide production by upregulating MKP-2 and dephosphorylating ERK. Int. Immunopharmacol. 75, 105787. https://doi.org/10.1016/j.intimp.2019.105787 (2019).
Kaplan, M. J. & Radic, M. Neutrophil extracellular traps: double-edged swords of innate immunity. J. Immunol. 189(6), 2689–2695. https://doi.org/10.4049/jimmunol.1201719 (2012).
Ermert, D. et al. Mouse neutrophil extracellular traps in microbial infections. J. Innate Immun. 1(3), 181–193. https://doi.org/10.1159/000205281 (2009).
Carmona-Rivera, C. & Kaplan, M. J. Induction and quantification of NETosis. Curr. Protoc. Immunol. 115, 14.41.1-14.41.14. https://doi.org/10.1002/cpim.16 (2016).
Acknowledgements
This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) within the National Institutes of Health (NIH). The contributions of the NIH authors are considered Works of the United States Government. The findings and conclusions presented in this paper are those of the authors and do not necessarily reflect the views of the NIH or the U.S. Department of Health and Human Services. We thank Jeff M. Reece (Advanced Light Microscopy & Image Analysis Core, NIDDK, NIH), Pradeep Dagur (Flow Cytometry Core at the National Heart, Lung, and Blood Institute, NIH), and Teruhiko Yoshida (NIDDK, NIH) for technical support.
Funding
Open access funding provided by the National Institutes of Health. This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) within the National Institutes of Health (NIH). The contributions of the NIH authors are considered Works of the United States Government. The findings and conclusions presented in this paper are those of the authors and do not necessarily reflect the views of the NIH or the U.S. Department of Health and Human Services. Open access funding provided by the National Institutes of Health
Author information
Authors and Affiliations
Contributions
Y.N., N.H., P.Y., and R.S. conceived and designed research; Y.N., D.G., N.H., and X.H. performed experiments; Y.N., D.G., and N.H. analyzed data; Y.N., P.Y., and R.S. interpreted results of experiments; Y.N., P.Y., and R.S. prepared figures; Y.N., D.G., N.H., P.Y., and R.S. drafted manuscript; Y.N., D.G., N.H., P.Y., and R.S. edited and revised manuscript; Y.N., D.G., N.H., X.H., P.Y., and R.S. approved final version of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.










Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Naito, Y., Goto, D., Hayase, N. et al. Peritoneal neutrophil extracellular traps contribute to septic AKI via peritoneal IL-17A and distant organ CXCL-1/ CXCL-2 pathway in abdominal sepsis. Sci Rep (2026). https://doi.org/10.1038/s41598-025-34770-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-34770-1