Abstract
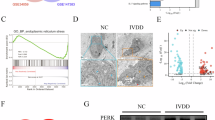
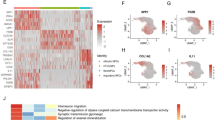
Intervertebral disc degeneration (IDD) is a multifactorially regulated age-related condition. Golgin B1 (GOLGB1) is a large Golgi-related transmembrane protein encoded by golgb1 gene. Since its discovery, GOLGB1 has been proved to be related to many diseases. However, its role in IDD is still unclear. The aim of this research was to examine whether GOLGB1 deficiency accelerates disc degeneration and to study its mechanism. We selected C57BL/6 male mice aged 8 weeks to isolate, culture and identify nucleus pulposus (NP) cells, and used a variety of experimental techniques, including plasmid transfection, western blotting (WB), real-time fluorescence quantitative PCR (RT-qPCR), EdU staining, Tunnel staining, CCK8 and flow cytometry (FCM) to detect the effects of GOLGB1 on the degeneration, proliferation and apoptosis of NP cells in vitro. Then, 8-week-old C57BL/6 male mice were selected for caudal vertebra acupuncture to establish IDD model, and the role and mechanism of GOLGB1 in the development of IDD were studied by HE staining, Safranin O-Fast Green staining, Alcian Blue staining and immunohistochemistry (IHC) staining. Our results demonstrated lower expression of GOLGB1 in human tissues with NP degeneration. Additionally, GOLGB1 expression was reduced in NP tissue from aging adult mice and IDD model mice. We further verified that GOLGB1 knockdown worsened NP cell degeneration, disturbed the composition of the extracellular matrix (ECM), and upset the harmony between anabolism and catabolism. Moreover, GOLGB1 knockdown inhibited the proliferation of NP cells and increased the apoptosis of NP cells. Mechanistically, GOLGB1 knockdown elevated p-ERK and p-P38 levels and activated the MAPK pathway. A MAPK signaling pathway inhibitor (SCH772984) strongly reversed this phenomenon and rescued the degeneration of NP cells. These findings suggest that GOLGB1 may be a potential new target for IDD. GOLGB1 is a protective factor against disc degeneration, and knockdown of GOLGB1 accelerates IDD by activating the MAPK pathway.
Similar content being viewed by others
Data availability
The data used to support the findings of this study will be available with the request to the corresponding author.
Abbreviations
- IDD:
-
Intervertebral disc degeneration
- GOLGB1:
-
Golgin B1
- NP:
-
Nucleus pulposus
- WB:
-
Western blotting
- RT-qPCR:
-
Real-time fluorescence quantitative PCR
- FCM:
-
Flow cytometry
- IHC:
-
Immunohistochemistry
- ECM:
-
Extracellular matrix
- LBP:
-
Low back pain
- BSA:
-
Bovine serum albumin
- FBS:
-
Fetal bovine serum
- EdU:
-
5-Ethynyl-2'-deoxyuridine
- CCK8:
-
Cell counting kit-8
References
Kadow, T., Sowa, G., Vo, N. & Kang, J. D. Molecular basis of intervertebral disc degeneration and herniations: what are the important translational questions? Clin. Orthop. Relat. Res. 473, 1903–1912. https://doi.org/10.1007/s11999-014-3774-8 (2015).
Khan, A. N. et al. Inflammatory biomarkers of low back pain and disc degeneration: a review. Ann. N Y Acad. Sci. 1410, 68–84. https://doi.org/10.1111/nyas.13551 (2017).
Maher, C., Underwood, M. & Buchbinder, R. Non-specific low back pain. Lancet 389, 736–747. https://doi.org/10.1016/s0140-6736(16)30970-9 (2017).
Hoy, D. et al. The global burden of low back pain: estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 73, 968–974. https://doi.org/10.1136/annrheumdis-2013-204428 (2014).
Hartvigsen, J. et al. What low back pain is and why we need to pay attention. Lancet 391, 2356–2367. https://doi.org/10.1016/s0140-6736(18)30480-x (2018).
Chen, S. et al. Global, regional and National burden of low back pain 1990–2019: A systematic analysis of the global burden of disease study 2019. J. Orthop. Translat. 32, 49–58. https://doi.org/10.1016/j.jot.2021.07.005 (2022).
Kamangar, F. et al. The global, regional, and National burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol. Hepatol. 5, 582–597. https://doi.org/10.1016/s2468-1253(20)30007-8 (2020).
Risbud, M. V. & Shapiro, I. M. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat. Rev. Rheumatol. 10, 44–56. https://doi.org/10.1038/nrrheum.2013.160 (2014).
Wang, J. et al. Tumor necrosis factor alpha- and interleukin-1beta-dependent induction of CCL3 expression by nucleus pulposus cells promotes macrophage migration through CCR1. Arthritis Rheum. 65, 832–842. https://doi.org/10.1002/art.37819 (2013).
Sakai, D. et al. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat. Commun. 3, 1264. https://doi.org/10.1038/ncomms2226 (2012).
Henriksson, H. B. & Brisby, H. Development and regeneration potential of the mammalian intervertebral disc. Cells Tissues Organs. 197, 1–13. https://doi.org/10.1159/000341153 (2013).
Vo, N. V. et al. Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J. 13, 331–341. https://doi.org/10.1016/j.spinee.2012.02.027 (2013).
Sivan, S. S., Wachtel, E. & Roughley, P. Structure, function, aging and turnover of Aggrecan in the intervertebral disc. Biochim. Biophys. Acta. 1840, 3181–3189. https://doi.org/10.1016/j.bbagen.2014.07.013 (2014).
Bergen, D. J. M., Stevenson, N. L., Skinner, R. E. H., Stephens, D. J. & Hammond, C. L. The golgi matrix protein Giantin is required for normal cilia function in zebrafish. Biol. Open. 6, 1180–1189. https://doi.org/10.1242/bio.025502 (2017).
Katayama, K. et al. Insertional mutation in the Golgb1 gene is associated with osteochondrodysplasia and systemic edema in the OCD rat. Bone 49, 1027–1036. https://doi.org/10.1016/j.bone.2011.08.001 (2011).
Lan, Y., Zhang, N., Liu, H., Xu, J. & Jiang, R. Golgb1 regulates protein glycosylation and is crucial for mammalian palate development. Development 143, 2344–2355. https://doi.org/10.1242/dev.134577 (2016).
Wang, J., Morita, Y., Mazelova, J. & Deretic, D. The Arf GAP ASAP1 provides a platform to regulate Arf4- and Rab11-Rab8-mediated ciliary receptor targeting. EMBO J. 31, 4057–4071. https://doi.org/10.1038/emboj.2012.253 (2012).
Katayama, K., Kuriki, M., Kamiya, T., Tochigi, Y. & Suzuki, H. Giantin is required for coordinated production of aggrecan, link protein and type XI collagen during chondrogenesis. Biochem. Biophys. Res. Commun. 499, 459–465. https://doi.org/10.1016/j.bbrc.2018.03.163 (2018).
Kerner, B. et al. Rare genomic variants link bipolar disorder with anxiety disorders to CREB-Regulated intracellular signaling pathways. Front. Psychiatry. 4, 154. https://doi.org/10.3389/fpsyt.2013.00154 (2013).
Ji-Hye et al. Mutations acquired by hepatocellular carcinoma recurrence give rise to an aggressive phenotype. Oncotarget 8, 22903–22916. https://doi.org/10.18632/oncotarget.14248 (2017).
Zhang, J. et al. TGF-beta1 suppresses CCL3/4 expression through the ERK signaling pathway and inhibits intervertebral disc degeneration and inflammation-related pain in a rat model. Exp. Mol. Med. 49, e379. https://doi.org/10.1038/emm.2017.136 (2017).
Bergmann, W. et al. Intervertebral disc degeneration in warmblood horses: Morphology, Grading, and distribution of lesions. Vet. Pathol. 55, 442–452. https://doi.org/10.1177/0300985817747950 (2018).
Li, Z. et al. Leptin downregulates Aggrecan through the p38-ADAMST pathway in human nucleus pulposus cells. PLoS One. 9, e109595. https://doi.org/10.1371/journal.pone.0109595 (2014).
Liu, C. et al. Resistin promotes intervertebral disc degeneration by upregulation of ADAMTS-5 through p38 MAPK signaling pathway. Spine (Phila Pa. 1976). 41, 1414–1420. https://doi.org/10.1097/BRS.0000000000001556 (2016).
Chen, X., Zhang, P. & Ma, X. Rab7 delays intervertebral disc degeneration through the Inhibition of the p38MAPK pathway. Biochem. Biophys. Res. Commun. 514, 835–841. https://doi.org/10.1016/j.bbrc.2019.04.184 (2019).
Wang, J. et al. TNF-alpha and IL-1beta promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated Aggrecan degradation through syndecan-4 in intervertebral disc. J. Biol. Chem. 286, 39738–39749. https://doi.org/10.1074/jbc.M111.264549 (2011).
Shen, B., Melrose, J., Ghosh, P. & Taylor, F. Induction of matrix metalloproteinase-2 and – 3 activity in ovine nucleus pulposus cells grown in three-dimensional agarose gel culture by interleukin-1beta: a potential pathway of disc degeneration. Eur. Spine J. 12, 66–75. https://doi.org/10.1007/s00586-002-0454-2 (2003).
Munro, S. The golgin coiled-coil proteins of the Golgi apparatus. Cold Spring Harb Perspect. Biol. https://doi.org/10.1101/cshperspect.a005256 (2011).
Alvarez, C., Garcia-Mata, R., Hauri, H. P. & Sztul, E. The p115-interactive proteins GM130 and Giantin participate in Endoplasmic reticulum-Golgi traffic. J. Biol. Chem. 276, 2693–2700. https://doi.org/10.1074/jbc.M007957200 (2001).
Wang, F., Cai, F., Shi, R., Wang, X. H. & Wu, X. T. Aging and age related stresses: a senescence mechanism of intervertebral disc degeneration. Osteoarthr. Cartil. 24, 398–408. https://doi.org/10.1016/j.joca.2015.09.019 (2016).
Zhang, J. et al. TNF-alpha enhances apoptosis by promoting Chop expression in nucleus pulposus cells: role of the MAPK and NF-kappaB pathways. J. Orthop. Res. 37, 697–705. https://doi.org/10.1002/jor.24204 (2019).
Cui, H. et al. Visfatin promotes intervertebral disc degeneration by inducing IL-6 expression through the ERK/JNK/p38 signalling pathways. Adipocyte 10, 201–215. https://doi.org/10.1080/21623945.2021.1910155 (2021).
Fang, W. et al. Wogonin mitigates intervertebral disc degeneration through the Nrf2/ARE and MAPK signaling pathways. Int. Immunopharmacol. 65, 539–549. https://doi.org/10.1016/j.intimp.2018.10.024 (2018).
Miao, D. & Zhang, L. Leptin modulates the expression of catabolic genes in rat nucleus pulposus cells through the mitogen-activated protein kinase and Janus kinase 2/signal transducer and activator of transcription 3 pathways. Mol. Med. Rep. 12, 1761–1768. https://doi.org/10.3892/mmr.2015.3646 (2015).
Wang, J. et al. BRD4 Inhibition regulates MAPK, NF-κB signals, and autophagy to suppress MMP‐13 expression in diabetic intervertebral disc degeneration. FASEB J. 33, 11555–11566. https://doi.org/10.1096/fj.201900703R (2019).
Acknowledgements
We thank the Shanxi key Laboratory of Bone and Soft Tissue Injury Repair of the Second Hospital of Shanxi Medical University for its experimental environment and equipment support.
Funding
Shanxi Province Higher Education “Billion Project” Science and Technology Guidance Project (BYJL030 and BYJL013); the Natural Science Foundation of Shanxi Province, China (202203021211040 and 20210302124670); Shanxi Provincial Science and Technology Cooperation and Exchange Special Project (202104041101023).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The study was designed by Y.-F. W., B. Z., and J. Y. The data collections were performed by J.-Y. T., Z.-Q. W., R.X. L., X.T. Q. and D.Q. H. The statistical analyses were performed by J.Y. T., N. S. and G.-H. H. The data interpretations were performed by Z.-Q. W. and Q.C. L. The literature searches were performed by all authors. The funds collection was provided by J. Y. and Y.-F. W. Finally, the first draft of the manuscript was written by J.-Y. T. and Z.-Q. W., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Second Hospital of Shanxi Medical University (approval number: 2021YX Number 019) and written informed consent was acquired from all participants. Animal procedures involving C57BL/6 mice were strictly authorized by the Ethics Committee of the Animal Transformation Center of Shanxi Medical University (approval number: DW2022053), which complies with the Guide for the Care and Use of Laboratory Animals, published by the United States National Institutes of Health (2011). All methods in the present study were designed and performed in accordance with ARRIVE guidelines and the other relevant guidelines and regulations.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tian, J., Wang, Z., Liu, R. et al. GOLGB1 deficiency accelerates intervertebral disc degeneration by activating the MAPK pathway. Sci Rep (2026). https://doi.org/10.1038/s41598-025-34797-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-34797-4