Abstract
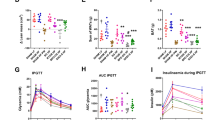
Obesity, defined as excessive fat accumulation that impairs health, is a global challenge with high mortality rates. Sibutramine, a centrally acting anorectic, was widely used in obesity treatment due to its effects on appetite control and weight loss. It represented a key step in anti-obesity pharmacotherapy before being withdrawn in many countries. This study evaluates how a high sibutramine dose (10 mg.kg.day−1 followed by 13 mg.kg.day−1) influences body weight, biochemical profiles, liver and intestinal health, and gut microbiota composition in rats subjected to a cafeteria diet for 16 weeks. Rats treated with sibutramine exhibited a 10.93% reduction in body weight and improved glycemic control compared to untreated groups. Sibutramine modulated lipid profiles by lowering total cholesterol and increasing HDL levels in controlled diet groups. The treatment attenuated liver damage markers (AST and ALT) and increased intestinal crypt depth, suggesting gut integrity. The elevated sibutramine dose led to distinct microbiota shifts, partially reversing cafeteria diet-induced dysbiosis by increasing SCFA-producing genera, including Bacillus, Marvinbryantia, and Bifidobacterium. However, dietary factors remained the dominant driver of microbial composition. These findings underscore the impact of sibutramine’s dosing on gut microbiota and reinforce its role in obesity-related health improvements.
Similar content being viewed by others
Data availability
The gut microbiota sequencing dataset is available under BioProject ID PRJNA1139136. The data can be accessed via the following link: https://www.ncbi.nlm.nih.gov/sra/PRJNA1139227.
References
WHO. Obesity and overweight. (2016).
Michaelidou, M., Pappachan, J. M. & Jeeyavudeen, M. S. Management of diabesity: current concepts. World J. Diabetes. 14, 396–411. https://doi.org/10.4239/wjd.v14.i4.396 (2023).
Elmaleh-Sachs, A. et al. Obesity management in adults: a review. JAMA 330, 2000–2015. https://doi.org/10.1001/jama.2023.19897 (2023).
Asadi, A. et al. Obesity and gut-microbiota-brain axis: A narrative review. J. Clin. Lab. Anal. 36, e24420. https://doi.org/10.1002/jcla.24420 (2022).
Muller, T. D., Bluher, M., Tschop, M. H. & DiMarchi, R. D. Anti-obesity drug discovery: advances and challenges. Nat. Rev. Drug Discov. 21, 201–223. https://doi.org/10.1038/s41573-021-00337-8 (2022).
Gaskin, C. J. et al. Clinical practice guidelines for the management of overweight and obesity published internationally: A scoping review. Obes. Rev. 25, e13700. https://doi.org/10.1111/obr.13700 (2024).
Raineri, S. et al. Pharmacologically induced weight loss is associated with distinct gut Microbiome changes in obese rats. BMC Microbiol. 22, 91. https://doi.org/10.1186/s12866-022-02494-1 (2022).
Tamayo-Trujillo, R. et al. Molecular mechanisms of semaglutide and liraglutide as a therapeutic option for obesity. Front. Nutr. 11, 1398059. https://doi.org/10.3389/fnut.2024.1398059 (2024).
Rodriguez, P. J. et al. Semaglutide vs Tirzepatide for weight loss in adults with overweight or obesity. JAMA Intern. Med. 184, 1056–1064. https://doi.org/10.1001/jamainternmed.2024.2525 (2024).
Coutinho, W. & Halpern, B. Pharmacotherapy for obesity: moving towards efficacy improvement. Diabetol. Metab. Syndr. 16, 6. https://doi.org/10.1186/s13098-023-01233-4 (2024).
Arterburn, D. E., Crane, P. K. & Veenstra, D. L. The efficacy and safety of sibutramine for weight loss: a systematic review. Arch. Intern. Med. 164, 994–1003. https://doi.org/10.1001/archinte.164.9.994 (2004).
Gorgojo-Martinez, J. J. et al. Clinical recommendations to manage Gastrointestinal adverse events in patients treated with GLP-1 receptor agonists: a multidisciplinary expert consensus. J. Clin. Med. 12, 145. https://doi.org/10.3390/jcm12010145 (2022).
Nisoli, E. & Carruba, M. O. An assessment of the safety and efficacy of sibutramine, an anti-obesity drug with a novel mechanism of action. Obes. Rev. 1, 127–139. https://doi.org/10.1046/j.1467-789x.2000.00020.x (2000).
Quesada-Vazquez, S. et al. Microbiota dysbiosis and gut barrier dysfunction associated with non-alcoholic fatty liver disease are modulated by a specific metabolic cofactors’ combination. Int. J. Mol. Sci. 23, 13675. https://doi.org/10.3390/ijms232213675 (2022).
Zhao, B. et al. Gut microbial dysbiosis is associated with metabolism and immune factors in liver fibrosis mice. Int. J. Biol. Macromol. 258, 129052. https://doi.org/10.1016/j.ijbiomac.2023.129052 (2024).
Vich Vila, A. et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat. Commun. 11, 362. https://doi.org/10.1038/s41467-019-14177-z (2020).
Noor, J., Chaudhry, A., Batool, S., Noor, R. & Fatima, G. Exploring the impact of the gut Microbiome on obesity and weight loss: a review Article. Cureus 15, e40948. https://doi.org/10.7759/cureus.40948 (2023).
Wu, Y. et al. Gut microbiota associated with appetite suppression in high-temperature and high-humidity environments. EBioMedicine 99, 104918. https://doi.org/10.1016/j.ebiom.2023.104918 (2024).
Bray, G. A. et al. Sibutramine produces dose-related weight loss. Obes. Res. 7, 189–198. https://doi.org/10.1002/j.1550-8528.1999.tb00701.x (1999).
Hanotin, C., Thomas, F., Jones, S. P., Leutenegger, E. & Drouin, P. Efficacy and tolerability of sibutramine in obese patients: a dose-ranging study. Int. J. Obes. Relat. Metab. Disord. 22, 32–38. https://doi.org/10.1038/sj.ijo.0800540 (1998).
Smith, D. L. Jr., Robertson, H. T., Desmond, R. A., Nagy, T. R. & Allison, D. B. No compelling evidence that sibutramine prolongs life in rodents despite providing a dose-dependent reduction in body weight. Int. J. Obes. (Lond). 35, 652–657. https://doi.org/10.1038/ijo.2010.247 (2011).
James, W. P. et al. Effect of sibutramine on weight maintenance after weight loss: a randomised trial. STORM study Group. Sibutramine trial of obesity reduction and maintenance. Lancet 356, 2119–2125. https://doi.org/10.1016/s0140-6736(00)03491-7 (2000).
Levin, B. E. & Dunn-Meynell, A. A. Sibutramine alters the central mechanisms regulating the defended body weight in diet-induced obese rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279, R2222–2228. https://doi.org/10.1152/ajpregu.2000.279.6.R2222 (2000).
Brown, M. et al. Sibutramine reduces feeding, body fat and improves insulin resistance in dietary-obese male Wistar rats independently of hypothalamic neuropeptide Y. Br. J. Pharmacol. 132, 1898–1904. https://doi.org/10.1038/sj.bjp.0704030 (2001).
Martire, S. I., Holmes, N., Westbrook, R. F. & Morris, M. J. Altered feeding patterns in rats exposed to a palatable cafeteria diet: increased snacking and its implications for development of obesity. PLoS One. 8, e60407. https://doi.org/10.1371/journal.pone.0060407 (2013).
Roberts, C. K., Berger, J. J. & Barnard, R. J. Long-term effects of diet on leptin, energy intake, and activity in a model of diet-induced obesity. J. Appl. Physiol. (1985). 93, 887–893. https://doi.org/10.1152/japplphysiol.00224.2002 (2002).
Ma, W., Wu, Y., Lin, X., Yang, L. & Huang, L. Amelioration of inflammatory bowel disease by Bifidobacterium animalis subsp. Lactis XLTG11 in combination with mesalazine. Front. Microbiol. 15, 1472776. https://doi.org/10.3389/fmicb.2024.1472776 (2024).
Siptroth, J. et al. Variation of butyrate production in the gut Microbiome in type 2 diabetes patients. Int. Microbiol. 26, 601–610. https://doi.org/10.1007/s10123-023-00324-6 (2023).
Ribeiro, F. M. et al. Limited effects of low-to-moderate aerobic exercise on the gut microbiota of mice subjected to a high-fat diet. Nutrients 11, 149. https://doi.org/10.3390/nu11010149 (2019).
Ribeiro, F. M. et al. Discontinuation of HIIT restores diabesity while retraining increases gut microbiota diversity. iScience 27, 110365. https://doi.org/10.1016/j.isci.2024.110365 (2024).
Del Bas, J. M. et al. Alterations in gut microbiota associated with a cafeteria diet and the physiological consequences in the host. Int. J. Obes. (Lond). 42, 746–754. https://doi.org/10.1038/ijo.2017.284 (2018).
de la Garza, A. L. et al. Characterization of the cafeteria diet as simulation of the human Western diet and its impact on the lipidomic profile and gut microbiota in obese rats. Nutrients 15, 86. https://doi.org/10.3390/nu15010086 (2022).
Gual-Grau, A., Guirro, M., Boque, N. & Arola, L. Physiological, metabolic and microbial responses to obesogenic cafeteria diet in rats: the impact of strain and sex. J. Nutr. Biochem. 117, 109338. https://doi.org/10.1016/j.jnutbio.2023.109338 (2023).
Tao, Z. & Wang, Y. The health benefits of dietary short-chain fatty acids in metabolic diseases. Crit. Rev. Food Sci. Nutr. 65, 1579-1592. https://doi.org/10.1080/10408398.2023.2297811 (2024).
Kaakoush, N. O. et al. Alternating or continuous exposure to cafeteria diet leads to similar shifts in gut microbiota compared to Chow diet. Mol. Nutr. Food Res. 61, 1500815. https://doi.org/10.1002/mnfr.201500815 (2017).
Neto, J. et al. Impact of cafeteria diet and n3 supplementation on the intestinal microbiota, fatty acids levels, neuroinflammatory markers and social memory in male rats. Physiol. Behav. 260, 114068. https://doi.org/10.1016/j.physbeh.2022.114068 (2023).
Xie, Y. et al. Impact of a high–fat diet on intestinal stem cells and epithelial barrier function in middle–aged female mice. Mol. Med. Rep. 21, 1133–1144. https://doi.org/10.3892/mmr.2020.10932 (2020).
Xu, J. & Dz Chen, J. Effects of sibutramine on gastric emptying, intestinal motility and rectal tone in dogs. Dig. Dis. Sci. 53, 155–162. https://doi.org/10.1007/s10620-007-9837-x (2008).
Masek, T., Barisic, J., Micek, V. & Starcevic, K. Cafeteria diet and high-fructose rodent models of NAFLD differ in the metabolism of important PUFA and palmitoleic acid without additional influence of sex. Nutrients 12, 3339. https://doi.org/10.3390/nu12113339 (2020).
Sampey, B. P. et al. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet. Obes. (Silver Spring). 19, 1109–1117. https://doi.org/10.1038/oby.2011.18 (2011).
Sabuncu, T., Nazligul, Y., Karaoglanoglu, M., Ucar, E. & Kilic, F. B. The effects of sibutramine and Orlistat on the ultrasonographic findings, insulin resistance and liver enzyme levels in obese patients with non-alcoholic steatohepatitis. Rom J. Gastroenterol. 12, 189–192 (2003).
Oberholzer, H. M., Bester, M. J. & van der Schoor, C. Rats on a high-energy diet showing no weight gain present with ultrastructural changes associated with liver fibrosis. Ultrastruct Pathol. 37, 267–272. https://doi.org/10.3109/01913123.2013.790527 (2013).
Lean, M. E. Sibutramine - a review of clinical efficacy. Int. J. Obes. Relat. Metab. Disord 21, S30–6. (1997).
McNeely, W., Goa, K. L. & Sibutramine A review of its contribution to the management of obesity. Drugs 56, 1093–1124. https://doi.org/10.2165/00003495-199856060-00019 (1998).
Hildebrand, F. et al. Inflammation-associated enterotypes, host genotype, cage and inter-individual effects drive gut microbiota variation in common laboratory mice. Genome Biol. 14, R4. https://doi.org/10.1186/gb-2013-14-1-r4 (2013).
van der Schoor, C., Oberholzer, H. M., Bester, M. J. & van Rooy, M. J. The effect of sibutramine, a serotonin-norepinephrine reuptake inhibitor, on platelets and fibrin networks of male Sprague-Dawley rats: a descriptive study. Ultrastruct Pathol. 38, 399–405. https://doi.org/10.3109/01913123.2014.946635 (2014).
Ramanathan, R. et al. Role of human wharton’s jelly derived mesenchymal stem cells (WJ-MSCs) for rescue of d-galactosamine induced acute liver injury in mice. J. Clin. Exp. Hepatol. 7, 205–214. https://doi.org/10.1016/j.jceh.2017.03.010 (2017).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH image to imageJ: 25 years of image analysis. Nat. Methods. 9, 671–675. https://doi.org/10.1038/nmeth.2089 (2012).
Jesus, P. et al. Alteration of intestinal barrier function during activity-based anorexia in mice. Clin. Nutr. 33, 1046–1053. https://doi.org/10.1016/j.clnu.2013.11.006 (2014).
Corp., I. IBM SPSS Statistics for Windows Version 27.0. Armonk, NY: IBM Corp (2020).
Kuczynski, J. et al. Using QIIME to analyze 16S rRNA gene sequences from microbial communities. Curr. Protoc. Bioinf. 27 1E–5. https://doi.org/10.1002/0471250953.bi1007s36 (2011).
Callahan, B. J. et al. DADA2: High-resolution sample inference from illumina amplicon data. Nat. Methods. 13, 581–583. https://doi.org/10.1038/nmeth.3869 (2016).
Mirarab, S., Nguyen, N. & Warnow, T. SEPP: SATe-enabled phylogenetic placement. Pac. Symp. Biocomput. https://doi.org/10.1142/9789814366496_0024 (2012).
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–596. https://doi.org/10.1093/nar/gks1219 (2013).
Chong, J., Liu, P., Zhou, G. & Xia, J. Using Microbiomeanalyst for comprehensive statistical, functional, and meta-analysis of Microbiome data. Nat. Protoc. 15, 799–821. https://doi.org/10.1038/s41596-019-0264-1 (2020).
Acknowledgements
This study was supported by Coordenação de Aperfeiçoamento de Pessoal e Nı́vel Superior (CAPES), Fundação de Apoio ao Desenvolvimento de Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul (FUNDECT), Conselho Nacional de Desenvolvimento Cientı́fico e Tecnológico (CNPq), Financiadora de Estudos e Projetos (FINEP) and Fundação de Apoio à Pesquisa do Distrito Federal (FAPDF).
Author information
Authors and Affiliations
Contributions
H.K.L., O.L.F. conceived the study and designed experiments.; F.M.R., H.K.L., C.F.A.R., and O.L.F. developed methodology and analyzed the data; H.K.L. and C.F.A.R. performed the experiments work; O.N.S. performed histological assessment.; G.M., N.C., R.W.P., and A.P.C. helped with data analysis.; F.M.R., O.L.F. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ribeiro, F.M., Lima, H.K., Ribeiro, C.F.A. et al. Sibutramine high-doses effects in cafeteria diet-induced obese rats. Sci Rep (2026). https://doi.org/10.1038/s41598-026-35214-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-35214-0