Abstract
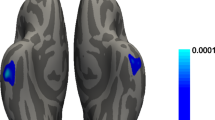
As the symptom of rapid eye movement sleep behavior disorder (RBD) is paroxysmal during sleeping, the disruptions of dynamic functional connectivity (FC) between thalamic subnuclei and the cortex may play a critical role in Parkinson’s disease (PD). A total of 35 PD patients with probable RBD (PD-pRBD) and 40 PD patients without probable RBD (PD-npRBD) and 41 healthy controls were enrolled. All participants underwent functional magnetic resonance imaging scan and clinical assessment. Altered dynamic FC between bilateral 14 thalamic nuclei and cortex was calculated. Although the RBDSQ demonstrates high diagnostic accuracy, polysomnographic validation would strengthen diagnostic certainty and enable more precise phenotyping of RBD severity. However, the current limitation of RBD diagnosis-relying solely on RBDSQ scores without polysomnographic confirmation-does not inherently compromise the outcome. PD-pRBD showed greater FC fluctuations between: (1) bilateral mediodorsal lateral parvocellular (MDL) nuclei and cerebellar anterior lobe (CAL); (2) right pulvinar lateral nucleus and left calcarine cortex; and (3) right ventral posterolateral nucleus and right cerebellum. Conversely, dynamic FC between the left pulvinar medial nucleus (PuM) and left superior parietal lobule, and between the right PuM nucleus and right inferior parietal lobule, were elevated in PD-npRBD. There was a positive correlation between the dynamic FC of bilateral MDL nuclei and the CAL with clinical severity within the PD-pRBD. Alterations in thalamocortical dynamic FC may be a potential biomarker for monitoring the paroxysmal nature of RBD in PD.
Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article.
References
Bruno, V. et al. Rapid eye movement sleep behavior disorder in Parkinson’s disease: A survey-based study. Can. J. Neurol. Sci. 50, 703–709. https://doi.org/10.1017/cjn.2022.291 (2023).
Cao, R. et al. Cross-sectional and longitudinal associations between probable rapid eye movement sleep behavior disorder and impulse control disorders in Parkinson’s disease. Eur. J. Neurol. 27, 757–763. https://doi.org/10.1111/ene.14177 (2020).
Jones, B. M. & McCarter, S. J. Rapid eye movement sleep behavior disorder: clinical presentation and diagnostic criteria. Sleep. Med. Clin. 19, 71–81. https://doi.org/10.1016/j.jsmc.2023.10.004 (2024).
Peever, J. & Fuller, P. M. The biology of REM sleep. Curr. Biol. 27, R1237–r1248. https://doi.org/10.1016/j.cub.2017.10.026 (2017).
Dirkx, M. F. et al. Dopamine controls Parkinson’s tremor by inhibiting the cerebellar thalamus. Brain 140, 721–734. https://doi.org/10.1093/brain/aww331 (2017).
Li, M. G. et al. Structural and functional thalamic changes in Parkinson’s disease with mild cognitive impairment. J. Magn. Reson. Imaging. 52, 1207–1215. https://doi.org/10.1002/jmri.27195 (2020).
Chen, M. et al. Structural and functional brain alterations in patients with idiopathic rapid eye movement sleep behavior disorder. J. Neuroradiol. 49, 66–72. https://doi.org/10.1016/j.neurad.2020.04.007 (2022).
Holtbernd, F. et al. Convergent patterns of structural brain changes in rapid eye movement sleep behavior disorder and Parkinson’s disease on behalf of the German rapid eye movement sleep behavior disorder study group. Sleep 44 https://doi.org/10.1093/sleep/zsaa199 (2021).
Klein, J. C. et al. The tremor network targeted by successful VIM deep brain stimulation in humans. Neurology 78, 787–795. https://doi.org/10.1212/WNL.0b013e318249f702 (2012).
Tucker, H. R. et al. Deep brain stimulation of the ventroanterior and ventrolateral thalamus improves motor function in a rat model of Parkinson’s disease. Exp. Neurol. 317, 155–167. https://doi.org/10.1016/j.expneurol.2019.03.008 (2019).
Lim, J. S. et al. Neural substrates of rapid eye movement sleep behavior disorder in Parkinson’s disease. Parkinsonism Relat. Disord. 23, 31–36. https://doi.org/10.1016/j.parkreldis.2015.11.027 (2016).
Boucetta, S. et al. Structural brain alterations associated with rapid eye movement sleep behavior disorder in Parkinson’s disease. Sci. Rep. 6, 26782. https://doi.org/10.1038/srep26782 (2016).
Sarasso, E. et al. Gait analysis and magnetic resonance imaging characteristics in patients with isolated rapid eye movement sleep behavior disorder. Mov. Disord. 39, 1567–1577. https://doi.org/10.1002/mds.29911 (2024).
Bourgouin, P. A. et al. Neuroimaging of rapid eye movement sleep behavior disorder. Int. Rev. Neurobiol. 144, 185–210. https://doi.org/10.1016/bs.irn.2018.10.006 (2019).
Rolinski, M. et al. Basal ganglia dysfunction in idiopathic REM sleep behaviour disorder parallels that in early Parkinson’s disease. Brain 139, 2224–2234. https://doi.org/10.1093/brain/aww124 (2016).
Preti, M. G., Bolton, T. A. & Van De Ville, D. The dynamic functional connectome: State-of-the-art and perspectives. Neuroimage 160, 41–54. https://doi.org/10.1016/j.neuroimage.2016.12.061 (2017).
Zhu, D. M. et al. Cerebellar-cerebral dynamic functional connectivity alterations in major depressive disorder. J. Affect. Disord. 275, 319–328. https://doi.org/10.1016/j.jad.2020.06.062 (2020).
Cao, Y. et al. Abnormal dynamic functional connectivity changes correlated with non-motor symptoms of Parkinson’s disease. Front. Neurosci. 17, 1116111. https://doi.org/10.3389/fnins.2023.1116111 (2023).
Engels, G., Vlaar, A., McCoy, B., Scherder, E. & Douw, L. Dynamic functional connectivity and symptoms of Parkinson’s disease: A resting-state fMRI study. Front. Aging Neurosci. 10, 388. https://doi.org/10.3389/fnagi.2018.00388 (2018).
Navalpotro-Gomez, I. et al. Disrupted salience network dynamics in Parkinson’s disease patients with impulse control disorders. Parkinsonism Relat. Disord. 70, 74–81. https://doi.org/10.1016/j.parkreldis.2019.12.009 (2020).
Hughes, A. J., Daniel, S. E., Kilford, L. & Lees, A. J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry. 55, 181–184. https://doi.org/10.1136/jnnp.55.3.181 (1992).
Chahine, L. M. et al. Longitudinal changes in cognition in early Parkinson’s disease patients with REM sleep behavior disorder. Parkinsonism Relat. Disord. 27, 102–106. https://doi.org/10.1016/j.parkreldis.2016.03.006 (2016).
Tomlinson, C. L. et al. Systematic review of Levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 25, 2649–2653. https://doi.org/10.1002/mds.23429 (2010).
Yan, C. G., Wang, X. D., Zuo, X. N. & Zang, Y. F. DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics. 14, 339–351. https://doi.org/10.1007/s12021-016-9299-4 (2016).
Ashburner, J. A fast diffeomorphic image registration algorithm. Neuroimage 38, 95–113. https://doi.org/10.1016/j.neuroimage.2007.07.007 (2007).
Rolls, E. T., Huang, C. C., Lin, C. P., Feng, J. & Joliot, M. Automated anatomical labelling atlas 3. Neuroimage 206, 116189. https://doi.org/10.1016/j.neuroimage.2019.116189 (2020).
Wang, S. et al. More than just static: dynamic functional connectivity changes of the thalamic nuclei to cortex in Parkinson’s disease with freezing of gait. Front. Neurol. 12, 735999. https://doi.org/10.3389/fneur.2021.735999 (2021).
Hutchison, R. M. et al. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage 80, 360–378. https://doi.org/10.1016/j.neuroimage.2013.05.079 (2013).
Choe, A. S. et al. Comparing test-retest reliability of dynamic functional connectivity methods. Neuroimage 158, 155–175. https://doi.org/10.1016/j.neuroimage.2017.07.005 (2017).
Leonardi, N. & Van De Ville, D. On spurious and real fluctuations of dynamic functional connectivity during rest. Neuroimage 104, 430–436. https://doi.org/10.1016/j.neuroimage.2014.09.007 (2015).
Zalesky, A. & Breakspear, M. Towards a statistical test for functional connectivity dynamics. Neuroimage 114, 466–470. https://doi.org/10.1016/j.neuroimage.2015.03.047 (2015).
Chakraborty, S., Ouhaz, Z., Mason, S. & Mitchell, A. S. Macaque parvocellular mediodorsal thalamus: dissociable contributions to learning and adaptive decision-making. Eur. J. Neurosci. 49, 1041–1054. https://doi.org/10.1111/ejn.14078 (2019).
Phillips, J. M., Kambi, N. A., Redinbaugh, M. J., Mohanta, S. & Saalmann, Y. B. Disentangling the influences of multiple thalamic nuclei on prefrontal cortex and cognitive control. Neurosci. Biobehav Rev. 128, 487–510. https://doi.org/10.1016/j.neubiorev.2021.06.042 (2021).
Stoodley, C. J. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum 11, 352–365. https://doi.org/10.1007/s12311-011-0260-7 (2012).
Cunchillos, J. D. & De Andrés, I. Participation of the cerebellum in the regulation of the sleep-wakefulness cycle. Results in cerebellectomized cats. Electroencephalogr. Clin. Neurophysiol. 53, 549–558. https://doi.org/10.1016/0013-4694(82)90067-0 (1982).
de Andrés, I., Garzón, M. & Reinoso-Suárez, F. Functional anatomy of non-REM sleep. Front. Neurol. 2, 70. https://doi.org/10.3389/fneur.2011.00070 (2011).
Pedroso, J. L. et al. Sleep disorders in cerebellar ataxias. Arq. Neuropsiquiatr. 69, 253–257. https://doi.org/10.1590/s0004-282x2011000200021 (2011).
Szirmai, I. [Pulvinar]. Neuropsychopharmacol. Hung. 15, 19–26 (2013).
Zhou, H., Schafer, R. J. & Desimone, R. Pulvinar-cortex interactions in vision and attention. Neuron 89, 209–220. https://doi.org/10.1016/j.neuron.2015.11.034 (2016).
Huang, A. W. & Barber, A. D. Development of lateral pulvinar resting state functional connectivity and its role in attention. Cortex 136, 77–88. https://doi.org/10.1016/j.cortex.2020.12.004 (2021).
Gorgoni, M. et al. The distinctive sleep pattern of the human calcarine cortex: a stereo-electroencephalographic study. Sleep. 44 https://doi.org/10.1093/sleep/zsab026 (2021).
Fernandez, L. M. J. & Lüthi, A. Sleep spindles: mechanisms and functions. Physiol. Rev. 100, 805–868. https://doi.org/10.1152/physrev.00042.2018 (2020).
Wolff, M. & Vann, S. D. The cognitive thalamus as a gateway to mental representations. J. Neurosci. 39, 3–14. https://doi.org/10.1523/jneurosci.0479-18.2018 (2019).
David, F. et al. Essential thalamic contribution to slow waves of natural sleep. J. Neurosci. 33, 19599–19610. https://doi.org/10.1523/jneurosci.3169-13.2013 (2013).
Han, J. et al. The neural correlates of arousal: ventral posterolateral nucleus-global transient co-activation. Cell. Rep. 43, 113633. https://doi.org/10.1016/j.celrep.2023.113633 (2024).
Stiasny-Kolster, K. et al. The REM sleep behavior disorder screening questionnaire–a new diagnostic instrument. Mov. Disord. 22, 2386–2393. https://doi.org/10.1002/mds.21740 (2007).
Funding
The National Natural Science Foundation of China awarded competitive funding (Award Code: 82230040) to enable.
Author information
Authors and Affiliations
Contributions
Conceptualization, S.T. and J.L.; Methodology, S.T.; Software, S.T., S.W.and Y.Z.; Formal analysis, S.T.; Writing-review & editing, S.T.,Y.Z., M.N., X.C. and J.L.; Data curation, S.T.; Validation, Y.Z. and S.W.; Project administration, M.N. and X.C.; Visualization, S.W.; Investigation, X.C.; Resources, X.C. and S.W.; Funding acquisition, J.L. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Informed consent
Participants were informed about the study and informed consents were obtained prior to their participation.
Institutional review board statement
All experimental protocols were approved by the Ruijin Hospital Ethics Committee (affiliated with Shanghai Jiao Tong University School of Medicine), granting Protocol ID: 2018 − 101.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tan, S., Zhang, Y., Niu, M. et al. Dynamic thalamocortical functional connectivity disruptions in Parkinson’s disease with probable REM sleep behavior disorder. Sci Rep (2026). https://doi.org/10.1038/s41598-026-35415-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-35415-7