Abstract
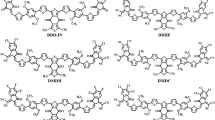
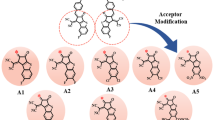
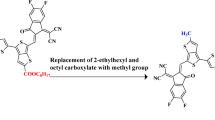
This study employs density functional theory (DFT) and time-dependent DFT (TD-DFT) to design and evaluate eight novel non-fullerene acceptors (NFAs) (G1–G8) for organic solar cells (OSCs). The molecules were engineered through strategic terminal group modification of a reference indacenodithiophene (IDT)-benzothidiazole (BT) based structure. All designed systems exhibit substantially reduced bandgaps (1.73–2.00 eV) and redshifted absorption profiles (λmax = 688–803 nm) compared to the reference molecule (REF), leading to enhanced light-harvesting capabilities (LHE = 0.988–0.998). Marcus charge transfer theory calculations revealed high hole hopping rates (Kh ≈ 10¹⁵ s⁻¹) and low reorganization energies (λh = 0.0031–0.0052 eV), indicating excellent charge transport properties. The comprehensive computational analysis projects outstanding photovoltaic performance with open-circuit voltage (VOC = 1.13–1.66 V), fill factor (FF = 0.8927–0.9205), and estimated power conversion efficiency (PCE = 22.8–37.0%) across the series. Among the designed systems, G7 demonstrates exceptional promise due to its optimal bandgap (1.73 eV), outstanding light-harvesting efficiency (LHE = 0.998), and the highest estimated short-circuit current (JSC = 31.2 mA/cm2), while G5 achieves the highest PCE (37.0%) through balanced photovoltaic parameters. The results establish terminal acceptor engineering as a highly effective strategy for developing high-performance organic photovoltaic materials, with G7 and G5 representing prime targets for experimental validation.
Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Peter, S. C. Reduction of CO2 to chemicals and fuels: A solution to global warming and energy crisis. ACS Energy Lett. 3(7), 1557–1561 (2018).
Höök, M. & Tang, X. Depletion of fossil fuels and anthropogenic climate change—A review. Energy Policy 52, 797–809 (2013).
Demirbaş, A. Global renewable energy resources. Energy Sources Part A Recov. Utili. Environ Effects 28(8), 779–792 (2006).
Sampaio, P. G. V. & González, M. O. A. Photovoltaic solar energy: Conceptual framework. Renew. Sustain. Energy Rev. 74, 590–601 (2017).
Sharma, S., Jain, K. K. & Sharma, A. Solar cells: In research and applications—a review. Mater. Sci. Appl. 6(12), 1145–1155 (2015).
Street, R. A., Northrup, J. E. & Krusor, B. S. Radiation induced recombination centers in organic solar cells. Phys. Rev. B Condens. Matter Mater. Phys. 85(20), 205211 (2012).
Shanmugam, M., Durcan, C. A. & Yu, B. Layered semiconductor molybdenum disulfide nanomembrane based Schottky-barrier solar cells. Nanoscale 4(23), 7399–7405 (2012).
Iftikhar, S. et al. Synthetic route for O, S-coordinated organotin (IV) aldehydes: Spectroscopic, computational, XRD, and antibacterial studies. Appl. Organomet. Chem. 38(8), e7581 (2024).
Servaites, J. D., Ratner, M. A. & Marks, T. J. Organic solar cells: A new look at traditional models. Energy Environ. Sci. 4(11), 4410–4422 (2011).
Khalil, A., Ahmed, Z., Touati, F., & Masmoudi, M. Review on organic solar cells. In 2016 13th International Multi-Conference on Systems, Signals & Devices (SSD) 342–353 (IEEE, 2016, March).
Yi, J., Zhang, G., Yu, H. & Yan, H. Advantages, challenges and molecular design of different material types used in organic solar cells. Nat. Rev. Mater. 9(1), 46–62 (2024).
Fukuda, K., Yu, K. & Someya, T. The future of flexible organic solar cells. Adv. Energy Mater. 10(25), 2000765 (2020).
Yan, C. et al. Non-fullerene acceptors for organic solar cells. Nat. Rev. Mater. 3(3), 1–19 (2018).
Camaioni, N. & Po, R. Pushing the envelope of the intrinsic limitation of organic solar cells. J. Phys. Chem. Lett. 4(11), 1821–1828 (2013).
Lu, C. J., Xu, Q., Feng, J. & Liu, R. R. The asymmetric Buchwald-Hartwig amination reaction. Angew. Chem. Int. Ed. 62(9), e202216863 (2023).
Zhan, C., Zhang, X. & Yao, J. New advances in non-fullerene acceptor based organic solar cells. RSC Adv. 5(113), 93002–93026 (2015).
Nielsen, C. B., Holliday, S., Chen, H. Y., Cryer, S. J. & McCulloch, I. Non-fullerene electron acceptors for use in organic solar cells. Acc. Chem. Res. 48(11), 2803–2812 (2015).
Khan, F. T., Ibrahim, M., Yousuf, A. & Ali, M. A. Extrusion of carbon with SON in heterocycles for enhanced static and dynamic hyperpolarizabilities and light harvesting efficiencies. Chem. Phys. 596, 112761 (2025).
Hedley, G. J., Ruseckas, A. & Samuel, I. D. Light harvesting for organic photovoltaics. Chem. Rev. 117(2), 796–837 (2017).
Duché, D. et al. Light harvesting in organic solar cells. Sol. Energy Mater. Sol. Cells 95, S18–S25 (2011).
Lee, J. K. & Yang, M. Progress in light harvesting and charge injection of dye-sensitized solar cells. Mater. Sci. Eng., B 176(15), 1142–1160 (2011).
Gao, W. et al. Simultaneously increasing open-circuit voltage and short-circuit current to minimize the energy loss in organic solar cells via designing asymmetrical non-fullerene acceptor. J. Mater. Chem. A 7(18), 11053–11061 (2019).
Yang, B. et al. Non-fullerene acceptors for large-open-circuit-voltage and high-efficiency organic solar cells. Mater. Today Nano 1, 47–59 (2018).
Qiu, B. et al. All-small-molecule nonfullerene organic solar cells with high fill factor and high efficiency over 10%. Chem. Mater. 29(17), 7543–7553 (2017).
Wang, X. et al. Precise fluorination of polymeric donors towards efficient non-fullerene organic solar cells with balanced open circuit voltage, short circuit current and fill factor. J. Mater. Chem. A 9(26), 14752–14757 (2021).
Wang, J. et al. Ultra-narrow bandgap non-fullerene organic solar cells with low voltage losses and a large photocurrent. J. Mater. Chem. A 6(41), 19934–19940 (2018).
Hai, J. et al. High-efficiency organic solar cells enabled by chalcogen containing branched chain engineering: Balancing short-circuit current and open-circuit voltage, enhancing fill factor. Adv. Funct. Mater. 33(19), 2213429 (2023).
Sun, Y. et al. Simultaneous enhancement of short-circuit current density, open circuit voltage and fill factor in ternary organic solar cells based on PTB7-Th: IT-M: PC71BM. Solar Energy Mater. Solar Cells 182, 45–51 (2018).
Chen, M. et al. Strategic molecular engineering of non-fused non-fullerene acceptors: Efficiency advances and mechanistic insight. Chem. Sci. 16(31), 14038–14080 (2025).
Huang, Y. et al. Mechanism of charge separation and transfer in doped third-component enhanced organic solar cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 347, 126969 (2025).
Dua, H., Paul, D. & Sarkar, U. A study on indolo [3, 2, 1-jk] carbazole donor-based dye-sensitized solar cells and effects from addition of auxiliary donors. Phys. Chem. Chem. Phys. 27(5), 2720–2731 (2025).
Ali, M. A. et al. Solvent-modulated second harmonic generation in N-alkylated thiohydantoin derivatives: Synthesis, characterization, and first-principle insights. RSC Adv. 15(44), 37325–37347 (2025).
Yousuf, A., Ullah, A., Hussain, S. Q. U., Ali, M. A. & Arshad, M. Spectroscopic studies and non-linear optical response through C/N replacement and modulation of electron donor/acceptor units on naphthyridine derivatives. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 329, 125582 (2025).
Kafourou, P. et al. One-step sixfold cyanation of benzothiadiazole acceptor units for air-stable high-performance n-type organic field-effect transistors. Angew. Chem. 133(11), 6035–6042 (2021).
Civalleri, B., Zicovich-Wilson, C. M., Valenzano, L. & Ugliengo, P. B3LYP-D3 augmented with an empirical dispersion term (B3LYP-D3-D*) as applied to molecular crystals. CrystEngComm 10(4), 405–410 (2008).
Ali, B. et al. Insight on the structural, electronic and optical properties of Zn, Ga-doped/dual-doped graphitic carbon nitride for visible-light applications. J. Mol. Graph. Model. 125, 108603 (2023).
Zulfiqar, R. et al. Design and prediction physicochemical properties of piperazinium and imidazolidinium based ionic liquids: A DFT and docking studies. ChemistrySelect 10(18), e202405487 (2025).
Arif, A. M., Yousaf, A., Xu, H. L. & Su, Z. M. Spectroscopic behavior, FMO, NLO and substitution effect of 2-(1H-Benzo [d] imidazole-2-ylthio)-No-substituted-acetamides: Experimental and theoretical approach. Dyes Pigm. 171, 107742 (2019).
Jia, H. L. et al. Efficient phenothiazine-ruthenium sensitizers with high open-circuit voltage (Voc) for high performance dye-sensitized solar cells. Dyes Pigments 180, 108454 (2020).
Scharber, M. C. et al. Design rules for donors in bulk-heterojunction solar cells—towards 10% energy-conversion efficiency. Adv. Mater. 18(6), 789–794 (2006).
Ibrahim, M. et al. Unlocking the potential of Indolo-Carbazole derivatives: First-principles insights into charge injection and optical switching applications. J. Phys. Chem. Solids 208(1), 113021 (2025).
Ibrahim, M., Khan, F. T., Xu, H. L. & Ali, M. A. Exploring the role of H-migration in the aromaticity, spectroscopic, photovoltaic and optical properties of planar heterocyclic compounds: A DFT study. Phys. Chem. Chem. Phys. 27(24), 12871–12885 (2025).
Ali, D., Ali, M. A., Yousuf, A. & Xu, H. L. From charge transfer to sustainability: A multifaceted DFT approach to ionic liquid design. FlatChem 52, 100899 (2025).
Tang, S. & Zhang, J. Design of donors with broad absorption regions and suitable frontier molecular orbitals to match typical acceptors via substitution on oligo (thienylenevinylene) toward solar cells. J. Comput. Chem. 33(15), 1353–1363 (2012).
Akhtar, M. et al. Tuning the NLO response of bis-cyclometalated iridium (III) complexes by modifying ligands: Experimental and structural DFT analysis. New J. Chem. 45(12), 5491–5496 (2021).
Paul, D. & Sarkar, U. Designing of PC31BM-based acceptors for dye-sensitized solar cell. J. Phys. Org. Chem. 36(12), e4419 (2023).
Bourass, M. et al. The computational study of the electronic and optoelectronics properties of new materials based on thienopyrazine for application in dye solar cells. J. Mater. Environ. Sci. 7(3), 700–712 (2016).
Saeed, M. U. et al. End-capped modification of Y-Shaped dithienothiophen [3, 2-b]-pyrrolobenzothiadiazole (TPBT) based non-fullerene acceptors for high performance organic solar cells by using DFT approach. Surf. Interfaces 30, 101875 (2022).
Ali, M. A. et al. Solvent-derived enhancement of electro-optic Pockels effect and second harmonic generation in heterocyclic/donor-acceptor functionalized α, β-unsaturated carbonyl compounds. J. Mol. Liquids 437(B), 128464 (2025).
Meng, Q., Hussain, S., He, Y., Lu, J. & Guerrero, J. M. Multi-timescale stochastic optimization for enhanced dispatching and operational efficiency of electric vehicle photovoltaic charging stations. Int. J. Electr. Power Energy Syst. 172, 111096 (2025).
Ullah, A. et al. Quantum chemical insights into metal-ion enhanced NLO response of a fluorescent probe for advanced sensing application. J. Fluoresc. 35, 1–21 (2025).
Zhao, Z. W. et al. A probe into underlying factors affecting utrafast charge transfer at Donor/IDIC interface of all-small-molecule nonfullerene organic solar cells. J. Photochem. Photobiol. A Chem. 375, 1–8 (2019).
Rana, M. et al. Biocompatible nitro group-based photosensitizer for AIE, hypoxia, and photodynamic therapy. Experimental and theoretical approach. J. Fluoresc. 35, 1–12 (2025).
Akhtar, M., Zhu, C., Ali, M. A., Ahmad, M. & Li, Z. A biocompatible core-shell nanoparticle encapsulating cyclometalated iridium (III) complexes and ultrasmall gold nanoclusters for ratiometric imaging of intracellular oxygen. Anal. Chem. 97(47), 26219–26229 (2025).
Grätzel, M. Solar energy conversion by dye-sensitized photovoltaic cells. Inorg. Chem. 44(20), 6841–6851 (2005).
Roohi, H. & Mohtamadifar, N. The role of the donor group and electron-accepting substitutions inserted in π-linkers in tuning the optoelectronic properties of D–π–A dye-sensitized solar cells: A DFT/TDDFT study. RSC Adv. 12(18), 11557–11573 (2022).
Kaifi, I. et al. Optimizing core modifications for high-performance D–A–D molecular systems: A multi-faceted study on NLO properties, solvent effects, charge transfer, and photovoltaic efficiency. Adv. Theory Simul. 8(8), 2500169 (2025).
Bibi, S. et al. Tailoring the donor moieties in TPA-based organic dyes for efficient photovoltaic, optical and nonlinear optical response properties. Int. J. Quant. Chem. 124(7), e27362 (2024).
UrRehman, S. et al. Designation of efficient diketopyrrolopyrrole based non-fullerene acceptors for OPVs: DFT study. Mater. Chem. Phys. 327, 129871 (2024).
Bibi, S. et al. Investigation analysis of optoelectronic and structural properties of cis-and trans-structures of azo dyes: density functional theory study. J. Phys. Organ. Chem. 34(6), e4183 (2021).
Qi, B. & Wang, J. Fill factor in organic solar cells. Phys. Chem. Chem. Phys. 15(23), 8972–8982 (2013).
Ma, W., Jiao, Y. & Meng, S. Predicting energy conversion efficiency of dye solar cells from first principles. J. Phys. Chem. C 118(30), 16447–16457 (2014).
Li, X. et al. Benzotriazole-based 3D four-arm small molecules enable 19.1% efficiency for PM6: Y6-based ternary organic solar cells. Angew. Chem. Int. Edn. 62(39), e202306847 (2023).
Wang, Z. et al. Dithienoquinoxalineimide-based polymer donor enables all-polymer solar cells over 19% efficiency. Angew. Chem. Int. Edn. 63(21), e202319755 (2024).
Dai, T. et al. Modulation of molecular quadrupole moments by phenyl side-chain fluorination for high-voltage and high-performance organic solar cells. J. Am. Chem. Soc. 147(5), 4631–4642 (2025).
Jiang, J. et al. ITIC surface modification to achieve synergistic electron transport layer enhancement for planar-type perovskite solar cells with efficiency exceeding 20%. J. Mater. Chem. A 5(20), 9514–9522 (2017).
Sağlamkaya, E. et al. What is special about Y6: The working mechanism of neat Y6 organic solar cells. Mater. Horizons 10(5), 1825–1834 (2023).
Cao, J., Yi, L., Zhang, L., Zou, Y. & Ding, L. Wide-bandgap polymer donors for non-fullerene organic solar cells. J. Mater. Chem. A 11(1), 17–30 (2023).
Acknowledgements
The researchers would like to thank the Deanship of Scientific Research, Qassim University for funding the publication of this study.
Funding
The authors declare that no funding was received to support this research.
Author information
Authors and Affiliations
Contributions
AG: Formal Analysis, Writing - Original draft preparation, Formal Analysis. AY: Visualization, Validation, Investigation. MZQ: Validation, Resources, Project administration. MAA: Conception, Supervision, Visualization, Writing - Original draft preparation, Writing - Review & Editing. MA: Methodology, Writing - Review & Editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Not Applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ghaffar, A., Yousuf, A., Qureshi, M.Z. et al. DFT study of benzothiadiazole based small molecules for high efficiency organic photovoltaics. Sci Rep (2026). https://doi.org/10.1038/s41598-026-35432-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-35432-6