Abstract
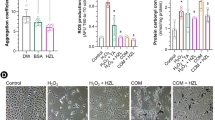
Nephrolithiasis (kidney stone) is a global health concern with multifactorial origins, including genetic, environmental, dietary, and metabolic factors. This study investigates the therapeutic potential of Annona muricata (AM) leaf extract in mitigating nephrolithiasis-induced electrolyte imbalance, antioxidant depletion, and histopathological alterations in an ethylene glycol (EG)-challenged rat model by modulating Na+/K+-ATPase and Ca2+/Mg2+-ATPase activities. Twenty-five male rats were randomly assigned to five experimental groups: 0.75% v/v ethylene glycol in drinking water for 28 days in the other groups, except the control (NC) group. The control (NC) and ethylene glycol-induced (EG) groups received distilled water, while other treatment groups were administered Zyloric (2 mg/kg/day), low-dose AM (200 mg/kg/day; EG + AML), and high-dose AM (800 mg/kg/day; EG + AMH) for 21 days. EG-induced disturbances in magnesium, sodium, potassium, chloride and calcium concentrations were effectively counteracted by both AM doses (200 mg/kg and 800 mg/kg/day) and Zyloric, with restorative effects on calcium levels of 8.02%, 18.18%, and 8.53%, and on magnesium levels of 12.77%, 25.24%, and 22.44%, respectively. Our findings suggest that AM exerts nephro-restorative effects by modulating electrolyte balance, ameliorating nephrolithiasis-induced histopathological changes, and enhancing antioxidant defences, as well as Na+/K+-ATPase and Ca2+/Mg2+-ATPase activities. These results showed the potential of AM as a therapeutic candidate for kidney stone management and related renal dysfunctions. Conclusively, AM exhibited therapeutic potential against ethylene glycol (Nephrolithiasis)-induced electrolyte imbalance, antioxidant depletion, and histopathological changes.
Similar content being viewed by others
Data availability
No, I do not have any research data outside the submitted manuscript file.
References
Nafiu, M. O. & Ogunsola, I. J. Anti-nephrolithiatic evaluation of partitioned ethanol extract of Calotropis procera leaf in wistar rats. Jordan J. Biol. Sci. 16(1), 432–437 (2023).
Suresh, S., Singh, A. & Vellapandian, C. Preclinical evaluation of Hibiscus cannabinus Linn. in the treatment of urolithiasis and cholelithiasis. Digit. Chin. Med. 6(2), 189–197 (2023).
Ranasinghe, R., Mathai, M. & Zulli, A. Cytoprotective remedies for ameliorating nephrotoxicity induced by renal oxidative stress. Life Sci. 318, 121466 (2023).
Patel, A., Shah, H. & Gandhi, T. Saponin rich fraction of Bauhinia variegata Linn. ameliorates kidney stone formation in rats. Exploratory Anim. Med. Res. 12(1), 234–237 (2022).
Obafemi, T. O. Gallic and hesperidin ameliorate electrolyte imbalances in AlCl3-induced nephrotoxicity in wistar rats. Biochem. Res. Int. 2022(1), 6151684 (2022).
Olanrewaju, T. O. Nephrolithiasis-induced chronic kidney disease–a case report. Nigerian Stethosc. 4(2), 58–71 (2022).
Modou, N. et al. Epidemiology and composition of upper urinary tract lithiasis in Senegalese population: a multicenter retrospective study. Urolithiasis 52(1), 462–468 (2023).
Tannor, E. K., Chika, O. U. & Okpechi, I. G. The impact of low socioeconomic status on progression of chronic kidney disease in low-and lower middle-income countries. In Seminars in Nephrology. Vol. 42, No. 5, 151338. (2022).
Uwumiro, F. et al. Impact of frailty on clinical outcomes and resource utilisation of hospitalisations for renal stone surgery. World J. Urol. 41(9), 2519–2526 (2023).
Balogun, J. A. Emerging developments in traditional medicine practice in Nigeria. In The Nigerian Healthcare System: Pathway To Universal and High-Quality Health Care, 235–275 (Springer International Publishing, 2022).
Shuaibu, G. B., Ashikaa, B. A., Muhammad, B. Y., Bamidele, T. O. & Zaruwa, M. Z. Antiurolithiatic potential of Parkia biglobosa, Lannea humilis stem bark methanol extract and KO-888 tonic. International J. Recent. Res. Life Sci. (IJRRLS). 10(3), 1–8 (2023).
Oreagba, A. et al. Evaluation of the antimalarial effects of the leaf extract and fruit juice of Annona muricata against plasmodium Berghei infection in mice. Univ. Lagos J. Basic. Med. Sci. 1(2), 235–238 (2022).
Henry-Unaeze, H. N. & Daniel, C. F. Evaluation of the chemical composition and sensory properties of soursop (Annona muricata) and watermelon (Citrullus lanatus) fruit juices and blends. J. Dietitians Association Nigeria. 12, 5–12 (2021).
Oyebamiji, A. K. et al. Annona muricata L. – A plant with vast pharmaceutical prospective: phytochemistry and pharmacology. Adeleke Univ. J. Sci. (AUJS). 1(2), 538–554 (2022).
Mutakin, M. et al. Pharmacological activities of soursop (Annona muricata Lin). Molecules 27(4), 1201 (2022).
Rahman, S. A., Sulaimon, L. A., Arogundade, O. L. & Akinolye, O. A. Phytochemical profiling and GC-MS analysis of Annona muricata ethanol leaf extract as potential agent for nephrolithiasis management: An in-vitro and in-silico investigation. J. Res. Pharm. Sci. 11(4), 01–21 (2025).
Percie du Sert, N. et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J. Cereb. Blood Flow. Metabolism. 40(9), 1769–1777 (2020).
Ajeel, M. A. & Al-Mahdawi, Z. M. M. Evaluation the role of Trefoil Factor1 as early-stage biomarker in patients with nephrolithiasis. Tikrit J. Pure Sci. 23(9), 16–19 (2018).
Temiz, O. In vivo neurotoxic effects of emamectin benzoate in male mice: evaluation with enzymatic and biomolecular multi-biomarkers. Environ. Sci. Pollut. Res. 29(6), 8921–8932 (2022).
Chauhan, N. & Pundir, C. S. An amperometric uric acid biosensor based on multiwalled carbon nanotube–gold nanoparticle composite. Anal. Biochem. 413(2), 97–103 (2011).
Talke, H. & Schubert, G. E. Enzymatic determination of urea using the coupled urease-glutamate dehydrogenase enzyme system. Klinische Wochenschrift. 43(3), 174–175 (1965).
Jaffe, M. On the precipitate produced by picric acid in normal urine and on a new reaction of creatinine. J. Physiological Chem. 10, 391–400 (1886).
Doumas, B. T., Watson, W. A. & Biggs, H. G. Albumin standards and the measurement of serum albumin with bromcresol green. Clin. Chim. Acta. 31(1), 87–96 (1971).
Marklund, S. & Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 47(3), 469–474 (1974).
Rotruck, J. T. et al. Selenium: biochemical role as a component of glutathione peroxidase. Sci 179(4073), 588–590 (1973).
Sreelakshmy, V., Anbarasi, G. & Vishnupriya, B. Salicylic acid pre-treatment induced physiological and biochemical changes in Solanum lycopersicum L. under salinity stress. Notulae Scientia Biologicae. 13(2), 10917–10917 (2021).
Zebalah, A. M. & Hamza, N. M. Study on toxic effect of tartrazine pigment on oxidative stress in male albino rats. Biochem. Cell. Archives. 21(1), 45–59 (2021).
Ogueji, E., Nwani, C., Mbah, C., Iheanacho, S. & Nweke, F. Oxidative stress, biochemical, lipid peroxidation, and antioxidant responses in Clarias gariepinus exposed to acute concentrations of Ivermectin. Environ. Sci. Pollut. Res. 27(14), 16806–16815 (2020).
Olayode, O. A., Daniyan, M. O. & Olayiwola, G. Biochemical, hematological and histopathological evaluation of the toxicity potential of the leaf extract of Stachytarpheta cayennensis in rats. J. Traditional Complement. Med. 10(6), 544–554 (2020).
Chamniansawat, S., Suksridechacin, N. & Thongon, N. Current opinion on the regulation of small intestinal magnesium absorption. World J. Gastroenterol. 29(2), 332 (2023).
Mou, Y., Li, B., Hou, Y., Jia, R. & Zhu, J. Effect of chronic hydrogen peroxide exposure on ion transport in gills of common carp (Cyprinus carpio). Fishes 8(3), 134 (2023).
Susilo, J., Purwanto, B., Doewes, M. & Indarto, D. Calcium oxalate crystals: Epidemiology, causes, modeling of crystal formation and treatment management. J. Pharm. Sci. Res. 13(2), 118–123 (2021).
Kyaw, M. T. & Maung, Z. M. Hypokalemia-induced arrhythmia: a case series and literature review. Cureus. 14(3) (2022).
Iweka, F. K. et al. The effect of potash on liver function of wistar rats. Int. J. Basic. Appl. Innovative Res. 5(1), 13–20 (2016).
Fiorentini, D., Cappadone, C., Farruggia, G. & Prata, C. Magnesium: biochemistry, nutrition, detection, and social impact of diseases linked to its deficiency. Nutrients 13(4), 1136 (2021).
Brini, M., Ottolini, D., Calì, T. & Carafoli, E. Calcium in health and disease. Interrelations between Essent. Metal Ions Hum. Diseases, 81–137. (2013).
Singh, P., Harris, P. C., Sas, D. J. & Lieske, J. C. The genetics of kidney stone disease and nephrocalcinosis. Nat. Rev. Nephrol. 18(4), 224–240 (2022).
Sakr, M. F. Calcium: why is it important?. In Parathyroid Gland Disorders: Controversies and Debates, 47–80 (Springer International Publishing, 2022).
Diringer, M. Neurologic manifestations of major electrolyte abnormalities. Handb. Clin. Neurol. 141, 705–713 (2017).
Yousefi Ghale-Salimi, M., Eidi, M., Ghaemi, N. & Khavari-Nejad, R. A. Antiurolithiatic effect of the taraxasterol on ethylene glycol induced kidney calculi in male rats. Urolithiasis 46(5), 419–428 (2018).
McMahon, B. A., Novick, T. & Murray, P. T. Correction of water, electrolyte, and acid-base derangements by hemodialysis and derived techniques. In Critical Care Nephrology, 941–947. (2019).
Chakit, M. et al. Antiurolithiatic activity of aqueous extract of Ziziphus lotus on ethylene glycol-induced lithiasis in rats. Pharmacognosy J. 14(5), 231–239 (2022).
Yang, J. et al. A new perspective on GC-MS urinary metabolomics analysis and efficient risk assessment of urolithiasis: morning urine organic acid profiles. Kidney Blood Press. Res. 1–21. (2024).
Dong, C. et al. Understanding formation processes of calcareous nephrolithiasis in renal interstitium and tubule lumen. J. Cell. Mol. Med. 28(7), e18235 (2024).
Peake, M. & Whiting, M. Measurement of serum creatinine–current status and future goals. Clin. Biochemist Reviews. 27(4), 173 (2006).
Sumien, N., Shetty, R. A. & Gonzales, E. B. Creatine, creatine kinase, and aging. Biochemistry and cell biology of ageing: part I biomedical science, 145–168. (2018).
Gutiérrez-Peredo, G. B. et al. J.C.B.O The urine protein/creatinine ratio as a reliable indicator of 24-h urine protein excretion across different levels of renal function and proteinuria: the TUNARI prospective study. BMC Nephrol. 25(1), 418 (2024).
Zhong, C., Long, R. & Stewart, G. S. The role of rumen epithelial urea transport proteins in urea nitrogen salvage: A review. Anim. Nutr. 9, 304–313 (2022).
Kassy, C. W. et al. Effects of lead exposure on biomarkers of thyroid and renal function tests among panel beaters in Enugu Metropolis, Nigeria. Niger. J. Clin. Pract. 25(9), 1593–1600 (2022).
Chávez-Iñiguez, J. S., Navarro-Gallardo, G. J., Medina-González, R., Alcantar-Vallin, L. & García-García, G. Acute kidney injury caused by obstructive nephropathy. Int. J. Nephrol. 1, 8846622 (2020).
Gburek, J., Konopska, B. & Gołąb, K. Renal handling of albumin—from early findings to current concepts. Int. J. Mol. Sci. 22(11), 5809 (2021).
Rao, C. Y., Sun, X. Y. & Ouyang, J. M. Effects of physical properties of nano-sized hydroxyapatite crystals on cellular toxicity in renal epithelial cells. Mater. Sci. Engineering: C. 103, 109807 (2019).
Shamna, S., Jose, J., Shijikumar, A. & Riyaz Ahmed, A. A brief study of nephrotoxicity and nephroprotective agents. Indian J. Pharm. Biol. Res. 8(1), 9–13 (2020).
Hayes, J. D., Dinkova-Kostova, A. T. & Tew, K. D. Oxidative stress in cancer. Cancer cell. 38(2), 167–197 (2020).
Sánchez-Rodríguez, M. A. & Mendoza-Núñez, V. M. Oxidative stress indexes for diagnosis of health or disease in humans. Oxidative Med. Cell. Longev. 2019(1), 4128152 (2019).
Eadon, M. T. et al. Kidney histopathology and prediction of kidney failure: a retrospective cohort study. Am. J. Kidney Dis. 76(3), 350–360 (2020).
Acknowledgements
We gratefully recognise the contributions made by the study team and the entire laboratory staff of the Department of Biochemistry, Crescent University, Abeokuta.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
This is SA PhD research under the supervision of LA, OL, and OA, while OA collaborated and assisted with histology.
Corresponding author
Ethics declarations
Ethical approval
All animal procedures and handling complied with established ethical and regulatory guidelines, including: The National Research Council’s Guide for the Care and Use of Laboratory Animals, which provides fundamental principles for the ethical treatment of experimental animals, and the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines as outlined by Percie et al., 2020, ensuring transparency and reproducibility in animal research. Ethical approval was granted by the Crescent University Research Ethics Committee, with the research protocols officially sanctioned under reference CRESCENT/CS/BCH/PG/S41512005.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rahman, S.A., Sulaimon, L.A., Arogundade, O.L. et al. Therapeutic efficacy of Annona muricata in counteracting nephrolithiasis-induced electrolyte imbalance and antioxidant disruption in ethylene glycol-treated rats. Sci Rep (2026). https://doi.org/10.1038/s41598-026-35535-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-35535-0