Abstract
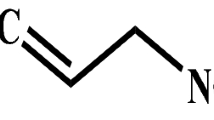
The current investigation aims to decipher the comparative anti-feeding and insecticidal potential of the purified form of Allyl isothiocyanate (AITC), a byproduct of glucosinolate hydrolysis, and three different mustard oils (black, brown and white) against Aulacophora foveicollis (red pumpkin beetle) adults and Spodoptera litura (tobacco cutworm) larvae. The in silico investigation undertakes the interaction of AITC with two anti-oxidant enzymes found in vertebrates, as AITC is also known to be an anti-oxidant and anti-cancerous compound for humans. The study also explores the AITC and protein-protein interaction among pepsin and mustard seed proteins, cruciferin and napin, as mustard contains abundant protein content but remains bio-unavailable due to presence of similar anti-nutritional factors like glucosinolates potentially having insect deterring potential. Through in vitro bioassays, it was found that the AITC was having higher insect anti-feeding potential (ranging from 74.63 to 88.22%) than the three mustard oils (ranging from 40.18 to 78.92%) against two insect pests studied in present investigation. The brown mustard oil showed LC50 as 602.23 mg/mL and 251.99 mg/mL, black mustard showed LC50 as 677.18 and 429.82 mg/mL while, white mustard oil showed LC50 as 835.21 mg/mL and 620.31 mg/mL against A. foveicollis and S. litura, respectively. The LC50 of AITC was observed to be higher, 3990 mg/mL (A. foveicollis) and 3690 mg/mL (S. litura). The in silico analysis revealed that leucine and aspartic acid are key mediators of Glutathione-S-transferase (GST)-AITC and Sulfonyl transferase (SULT)-AITC interactions in humans, respectively. The study also showed a stronger binding between human digestive enzyme (pepsin) and seed storage proteins of mustard (napin and cruciferin). In terms of insect deterrence, brown mustard oil outperformed both black and white mustard oils due to its higher glucosinolate concentration. It also showed greater efficacy than AITC at lower doses, likely because of the presence of various fatty acids in the oil that are known to deter insect pests. In the near future, the metabolites of mustard may be explored as an economical bio-control agent thus elaborating their role in replacing harmful synthetic insecticides that are a hurdle in attaining the goal of sustainable agriculture.
Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Lietzow, J. Biologically active compounds in mustard seeds: a toxicological perspective. Foods. 10(9), 2089 (2021).
Nguyen, V. P. T., Stewart, J., Lopez, M., Ioannou, I. & Allais, F. Glucosinolates: natural Occurrence, Biosynthesis, Accessibility, Isolation, Structures, and biological activities. Molecules 25 (19), 4537 (2020).
Barba, F. J. et al. Bioavailability of glucosinolates and their breakdown products: impact of processing. Front. Nutr. 3, 24 (2016).
Du, Y., Grodowitz, M. J. & Chen, J. Insecticidal and Enzyme Inhibitory Activities of Isothiocyanates against Red Imported Fire Ants, Solenopsis invicta. Biomolecules, 10(5), 716 (2020).
Cerda, R. et al. Primary and secondary yield losses caused by pests and diseases: assessment and modeling in coffee. PLoS One. 12 (1), e0169133 (2017).
Cates, R. G. Host plant predictability and the feeding patterns of oligophagous and insect herbivores. Oecologia 48 (3), 319–326 (1981).
Hardy, N. B. & Otto, S. P. Specialization and generalization in the diversification of phytophagous insects: tests of the musical chairs and Oscillation hypotheses. Proc. Royal Soc. B: Biol. Sci. 281 (1795), 20132960 (2014).
Shukla, G. S. & Singh, J. P. Studies on the rate of excretion of Aulacophora foviecollis Lucas., the red pumpkin beetle (Coleoptera: Chrysomelidae). Experientia, 26(3), 272 (1970).
Rolnik, A. & Olas, B. Vegetables from the cucurbitaceae family and their products: positive effect on human health. Nutrition 78, 110788 (2020).
Khan, M. M. H., Alam, M. Z., Rahman, M. M., Miah, M. I. H. & Hossain, M. M. Influence of weather factors on the incidence and distribution of pumpkin beetle infesting cucurbits. Bangladesh J. Agricultural Res. 37 (2), 361–367 (2012).
Vengateswari, G., Arunthirumeni, M. & Shivakumar, M. S. Effect of food plants on Spodoptera Litura (Lepidoptera: Noctuidae) larvae immune and antioxidant properties in response to Bacillus Thuringiensis infection. Toxicol. Rep. 7, 1428–1437 (2020).
Shekhawat, S. S., ShafiqAnsari, M. & Basri, M. Effect of host plants on life table parameters of Spodoptera Litura. Indian J. Pure Appl. Biosci. 6 (1), 324–332 (2018).
Garg, S., Nain, P., Joshi, R., Punetha, H. & Srivastava, R. M. Bio-efficacy of mustard seed extracts against Bihar hairy caterpillar Spilosoma obliqua (Erebidae: Lepidoptera) & assessment of mustard allelo-chemicals in response to mustard aphid Lipaphis erysimi (Aphididae: Hemiptera) infestation. South. Afr. J. Bot. 171, 156–163 (2024).
Mukhopadhyay, S. & Bhattacharyya, D. K. Colorimetric Estimation of allyl isothiocyanate content in mustard and rapeseed oils. Fette Seifen Anstrichm. 85 (8), 309–311 (1983).
Garg, S., Pant, U., Nain, P. & Punetha, H. Nutritional & Anti-Nutritional and Anti-Oxidative profiling of globally utilized diverse seed coat color mustards. Bioscience Forum – Int. J. 15 (4), 1261–1267 (2023).
Garg, S., Punetha, H., Chaudhary, D. & Srivastava, R. M. Comparative bio efficacy of allyl isothiocyanate and brown mustard oil against polyphagous insect pests Aulacophora foveicollis (Coleoptera: Chrysomelidae) and Spodoptera Litura (Lepidoptera: Noctuidae). International J. Trop. Insect Science (2025).
Das, G. et al. Glucosinolates and Omega-3 fatty acids from mustard seeds: phytochemistry and Pharmacology. Plants (Basel). 11 (17), 2290 (2022).
Cerón, D. A. C., de Alencar, E. R., Faroni, L. R. D., Silva, M. V. A. & Salvador, D. V. Toxicity of allyl isothiocyanate applied in systems with or without recirculation for controlling Sitophilus zeamais, Rhyzopertha dominica, and Tribolium castaneum in corn grains. J. Sci. Food. Agric. 103 (13), 6373–6382 (2023).
Gou, Y. P. et al. Responses of fungi maggot (Bradysia impatiens Johannsen) to allyl isothiocyanate and high CO2. Front. Physiol. 13, 879401 (2022).
Garg, S., Gairola, K., Punetha, H. & Gangola, S. An exploration of the biochemistry of mustard seed meals: A phytochemical and in Silico perspective. Foods 13 (24), 4130 (2024).
Ntone, E. et al. Napins and cruciferins in rapeseed protein extracts have complementary roles in structuring emulsion-filled gels. Food Hydrocoll. 125, 1–11 (2022).
Isman, B., Koul, O., Lucyzynski, A. & Kaminski, J. Insecticidal and antifeedant bioactivities of Neem oils and their relationship to Azadirachtin content. J. Agric. Food Chem. 38 (6), 1407–1411 (1997).
Duraipandiyan, V., Ignacimuthu, S. & Gabriel Paulraj, M. Antifeedant and larvicidal activities of Rhein isolated from the flowers of Cassia fistula L. Saudi J. Biol. Sci. 18 (2), 129–133 (2011).
Mawlong, I., Kumar, M. S. S., Gurung, B., Singh, K. H. & Singh, D. A simple spectrophotometric method for estimating total glucosinolates in mustard de-oiled cake. Int. J. Food Prop. 20 (S3), 3274–3281 (2017).
Tsao, R., Peterson, C. J. & Coats, J. R. Glucosinolate breakdown products as insect fumigants and their effect on carbon dioxide emission of insects. BMC Ecol. 2 (1), 1–7 (2002).
Garg, V. K. et al. K. MFPPI-multi FASTA ProtParam interface. Bioinformation 12 (2), 74–77 (2016).
Yoshikawa, N., Hutchison, G. R. & Fast Efficient fragment-based coordinate generation for open babel. J. Cheminform. 11 (1), 1–9 (2019).
Guterres, H. et al. CHARMM-GUI high‐throughput simulator for efficient evaluation of protein-ligand interactions with different force fields. Protein Sci. 31 (11), e4413 (2022).
Chatterjee, A., Roy, U. K. & Halder, D. Protein active site structure prediction strategy and algorithm. Int. J. Curr. Eng. Technol. 7 (3), 1092–1096 (2017).
Li, H. et al. Multi-ligand molecular docking, simulation, free energy calculations and wavelet analysis of the synergistic effects between natural compounds Baicalein and cubebin for the Inhibition of the main protease of SARS-CoV-2. J. Mol. Liq. 374, 121253 (2023).
Rahman, M. et al. In silico, molecular Docking and in vitro antimicrobial activity of the major rapeseed seed storage proteins. Front. Pharmacol. 11, 1340 (2020).
Yan, Y., Tao, H., He, J. & Huang, S. Y. The HDOCK server for integrated protein-protein Docking. Nat. Protoc. 15 (6), 1829–1852 (2020).
Souto, A. L. et al. Plant-Derived pesticides as an alternative to pest management and sustainable agricultural production: Prospects, applications and challenges. Molecules 26 (16), 4835 (2021).
Singh, S., Diksha, E. & Mahajan, E. Appraisal of growth inhibitory, biochemical and genotoxic effects of allyl isothiocyanate on different developmental stages of Zeugodacus cucurbitae (Coquillett) (Diptera: Tephritidae). Sci. Rep. 12 (1), 10363 (2022).
Wu, H., Liu, X., Yu, D., Zhang, X. & Feng, J. Effect of allyl isothiocyanate on ultra-structure and the activities of four enzymes in adult Sitophilus Zeamais. Pestic. Biochem. Physiol. 109, 12–17 (2014).
Worfel, R. C., Schneider, K. S. & Yang, T. C. Suppressive effect of allyl isothiocyanate on populations of stored grain insect pests. J. Food Process. Preserv. 21 (1), 9–19 (1997).
Santos, J. C., Faroni, L. R. A., Sousa, A. H. & Guedes, R. N. C. Fumigant toxicity of allyl isothiocyanate to populations of the red flour beetle Tribolium castaneum. J. Stored Prod. Res. 47 (4), 238–243 (2011).
Shi, C. H. et al. Control of bradysia Odoriphaga (Diptera: Sciaridae) with allyl isothiocyanate under field and greenhouse conditions. J. Econ. Entomol. 110 (3), 1127–1132 (2017).
Flor-Weiler, L. B. et al. Bioactivity of brassica seed meals and its compounds as ecofriendly larvicides against mosquitoes. Sci. Rep. 13 (1), 3936 (2023).
Arunthirumeni, M., Vinitha, G. & Shivakumar, M. S. Antifeeding and larvicidal activity of bioactive compounds isolated from entomopathogenic fungi Penicillium sp. for the control of agricultural and medically important insect pest (Spodoptera Litura and Culex quinquefasciatus). Parasitol. Int. 92, 102688 (2023).
Gupta, S., Chaudhary, A., Singh, S., Arora, S. & Sohal, S. K. Broccoli (Brassica Oleracea L. var. italica) cultivars, Palam Samridhi and Palam Vichitra affect the growth of Spodoptera Litura (Fabricius) (Lepidoptera: Noctuidae). Heliyon 7 (8), e07612 (2021).
Gujar, G. T. & Mehrotra, K. N. Biological activity of Neem against the red pumpkin beetle, Aulacophora foveicollis. Phytoparasitica 16 (4), 293–302 (1988).
Chandravadana, M. V. Identification of triterpenoid feeding deterrent of red pumpkin beetles (Aulacophora foveicollis) from Momordica Charantia. J. Chem. Ecol. 13 (7), 1689–1694 (1987).
Jan, Q. et al. Comparative conventional preventive strategies for insect pest of Okra. Saudi J. Biol. Sci. 29 (5), 3114–3121 (2022).
Pae, J. L., Faroni, L. R. D. A., Dhingra, O. D., Cecon, P. R. & Silva, T. A. Insecticidal fumigant action of mustard essential oil against Sitophilus Zeamais in maize grains. Crop Prot. 34, 56–58 (2012).
Konecka, E. et al. Insecticidal activity of Brassica alba mustard oil against lepidopteran pests Cydia pomonella (Lepidoptera: Tortricidae), Dendrolimus pini (Lepidoptera: Lasiocampidae), and Spodoptera exigua (Lepidoptera: Noctuidae). J. Plant Prot. Res. 58(2), 206–209 (2018).
Gupta, G., Kaur, G., Yadav, R. & Kumar, N. R. Repellent effects of aqueous extracts of mustard seeds Brassica juncea, on three major pests of horticultural crops. CJAAS 1 (1), 16–24 (2021).
House, H. L. & Graham, A. R. Capric acid blended into food stuff for control of an insect pest, Tribolium confusum (Coleoptera:tenebrionidae). Can. Entomol. 99 (9), 994–999 (1967).
Dasari, S., Ganjayi, M. S., Oruganti, L., Balaji, H. & Meriga, B. Glutathione S-transferases detoxify endogenous and exogenous toxic agents-minireview. J. Dairy. Veterinary Anim. Res. 5 (4), 1–3 (2017).
Bauer-Marinovic, M., Taugner, F., Florian, S. & Glatt, H. Toxicity studies with 5-hydroxymethylfurfural and its metabolite 5-sulphooxymethylfurfural in wild-type mice and Transgenic mice expressing human sulphotransferases 1A1 and 1A2. Arch. Toxicol. 86 (5), 701–711 (2012).
Pedersen, L. C., Yi, M., Pedersen, L. G. & Kaminski, A. M. From steroid and drug metabolism to glycobiology, using sulfotransferase structures to understand and tailor function. Drug Metab. Dispos. 50 (8), 1027–1041 (2022).
Sandamalika, W. G., Priyathilaka, T. T., Lee, S., Yang, H. & Lee, J. Immune and xenobiotic responses of glutathione S-Transferase theta (GST-θ) from marine invertebrate disk abalone (Haliotis discus discus): with molecular characterization and functional analysis. Fish Shellfish Immunol. 91, 159–171 (2019).
Bhutta, Z. A., Sadiq, K. & Aga, T. Protein digestion and bioavailability. Encyclopedia Hum. Nutr. 4, 116–122 (2013).
Perera, S. P., McIntosh, T. C. & Wanasundara, J. P. Structural properties of cruciferin and napin of Brassica napus (canola) show distinct responses to changes in pH and temperature. Plants, 5(3), 1–24 (2016).
Sarker, A. K., Saha, D., Begum, H., Zaman, A. & Rahman, M. M. Comparison of cake compositions, Pepsin digestibility and amino acids concentration of proteins isolated from black mustard and yellow mustard cakes. AMB Express. 5 (1), 22–26 (2015).
Haouel Hamdi, S., Hedjal-Chebheb, M., Kellouche, A., Khouja, M. L. & Boudabous, A. Mediouni Ben Jemaa, J. Management of three pests’ population strains from Tunisia and Algeria using Eucalyptus essential oils. Industrial Crops Prod. 74, 551–556 (2015).
Funding
No funding received.
Author information
Authors and Affiliations
Contributions
SG: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. HP: Supervision, Writing – review & editing. SaG: Supervision, Writing – review & editing AV.: Validation, Writing – review & editing. MT: Writing – review & editing. MJ: Supervision, Writing – review & editing. AM: Supervision, Writing – review & editing. FM: MT: Writing – review & editing, Supervision. AMA: Supervision, Writing – review & editing. MBBH: Funding acquisition, Validation, Supervision. NA: Funding acquisition, Writing – review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Garg, S., Punetha, H., Gangola, S. et al. Mustard derived compounds as insecticides and modulators of human metabolism. Sci Rep (2026). https://doi.org/10.1038/s41598-026-35536-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-35536-z