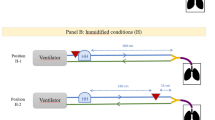
Abstract
Inhalation toxicity assessment relies heavily on quantifying both atmospheric concentration (AC) and region-specific internal dose (ID) within the respiratory tract, especially when comparing in vivo toxicity tests with in vitro respiratory cellular toxicity tests. However, for particulate substances, the precise relationship between ID and AC often becomes unclear due to variations in aerosol particle size distribution (PSD). This study aimed to investigate how the PSD of aerosols, generated from non-volatile and water-soluble biocides (benzalkonium chloride, didecyldimethylammonium chloride, polyhexamethylene guanidine phosphate, and paraquat), influences the relationship between AC and region-specific ID in the respiratory tract. Aerosols were generated at various concentrations using an ultrasonic humidifier in a 0.125 m³ acrylic chamber, with PSDs (0.01–10 μm) measured using real-time instruments. Regional deposition rates in the rat respiratory tract were subsequently calculated via the multi-path particle dosimetry (MPPD) model to estimate ID. As solution concentration increased, both AC and the mass median aerodynamic diameter (MMAD) also rose. The ID/AC ratio showed saturating increases in the head (H) and tracheobronchial (TB) regions, but a decreasing trend in the pulmonary (P) region, attributed to a reduction in fine particle fraction. A lognormal distribution-based analysis revealed that the TB region was most sensitive to changes in geometric standard deviation (GSD), whereas the H and P regions were primarily affected by MMAD variations. Crucially, a notable mismatch in ID/AC ratios was identified between the measured PSDs and those assumed under lognormal distribution models, underscoring the vital role of aerosol size characteristics in understanding respiratory deposition efficiency and dose metrics. These findings strongly suggest that a quantitative approach, incorporating measured PSDs, is essential for accurately interpreting and comparing inhalation exposure data across different toxicity evaluation systems. This goes beyond evaluations based solely on external concentrations, highlighting the necessity of detailed aerosol characterization.
Similar content being viewed by others
Data availability
Raw data not shown in the main body and the supplementary information are available upon request to the corresponding author.
Abbreviations
- AC:
-
Atmospheric concentration
- BAC:
-
Benzalkonium chloride
- DDAC:
-
Didecyldimethylammonium chloride
- DF:
-
Deposition fraction
- GSD:
-
geometric standard deviation
- H:
-
Head
- ID:
-
Internal dose
- MMAD:
-
Mass median aerodynamic diameter
- MPPD:
-
Multiple-Path Particle Dosimetry
- NAMs:
-
New approach methodologies
- NGRA:
-
Next generation risk assessment
- OECD:
-
Organization of Economic Co-operation and Development
- OPS:
-
Optical particle sizer
- P:
-
Pulmonary
- PHMG-p:
-
Polyhexamethylene guanidine phosphate
- PQ:
-
Paraquat
- PSD:
-
Particle size distribution
- SD:
-
Sprague-Dawley
- SMPS:
-
Scanning mobility particle sizer
- TB:
-
Tracheobronchial
References
Longhin, E. M. et al. Hazard assessment of nanomaterials: how to Meet the requirements for (next generation) risk assessment. Part. Fibre Toxicol. 21 (1), 1–31. https://doi.org/10.1186/s12989-024-00615-4 (2024).
Jeong, M. H. et al. Pre-validation of a Calu-3 epithelium cytotoxicity assay for predicting acute inhalation toxicity of chemicals. Toxicol. Vitro. 75, 105136. https://doi.org/10.1016/j.tiv.2021.105136 (2021).
Fernandez-Agudo, A. & Tarazona, J. V. A tiered next-generation risk assessment framework integrating toxicokinetics and NAM-based toxicodynamics:proof of concept case study using pyrethroids. Arch. Toxicol. 99, 2759–2781. https://doi.org/10.1007/s00204-025-04045-9 (2025).
de Ávila, R. I. et al. Evaluation of a non-animal toolbox informed by adverse outcome pathways for human inhalation safety. Front. Toxicol. 7, 1426132. https://doi.org/10.3389/ftox.2025.1426132 (2025).
Gijbels, M. J., Braeckman, B. P., Rogiers, V. & vanhaecke, T. Integration of next generation risk assessment (NGRA) tools and approaches in non-animal testing: a perspective on organotypic in vitro models. Arch. Toxicol. 97 (4), 1069–1090. https://doi.org/10.1016/j.impact.2024.100523 (2023).
Kim, J. W., Kim, H. S., Kim, H. R. & Chung, K. H. Next generation risk assessment of biocides (PHMG-p and CMIT/MIT)-induced pulmonary fibrosis using adverse outcome pathway-based transcriptome analysis. J. Hazard. Mater. 476, 134986. https://doi.org/10.1016/j.jhazmat.2024.134986 (2024).
Hinds, W. C. & Zhu, Y. Aerosol Technology: properties, behavior, and Measurement of Airborne Particles 3rd edn (Wiley, 2022).
Gehr, P. & Heyder, J. Particle-Lung Interactions (CRC, 2000).
Fröhlich, E. & Salar-Behzadi, S. Toxicological assessment of inhaled nanoparticles: role of in vivo, ex vivo, in vitro, and in Silico studies. Int. J. Mol. Sci. 15, 4795–4822. https://doi.org/10.3390/ijms15034795 (2014).
Miller, F. J., Asgharian, B., Schroeter, J. D. & Price, O. Improvements and additions to the multiple path particle dosimetry model. J. Aerosol Sci. 99, 14–26. https://doi.org/10.1016/j.jaerosci.2016.01.018 (2016).
Sain, A. E., Zook, J., Davy, B. M., Marr, L. C. & Dietrich, A. M. Size and mineral composition of airborne particles generated by an ultrasonic humidifier. Indoor Air. 28, 80–88. https://doi.org/10.1111/ina.12414 (2018).
Rodes, C., Smith, T., Crouse, R. & Ramachandran, G. Measurements of the sized distribution of aerosols produced by ultrasonic humidification. Aerosol Sci. Technol. 1990;13:220–9. (1990). https://doi.org/10.1080/02786829008959440
Lee, J-H., Kim, Y-H. & Kwon, J-H. Fatal misuse of humidifier disinfectants in korea: importance of screening risk assessment and implications for management of chemicals in consumer products. Environ. Sci. Technol. 46, 2498–2500. https://doi.org/10.1021/es300567j (2012).
Ji, J. H. & Yu, I. J. Estimation of human equivalent exposure from rat inhalation toxicity study of silver nanoparticles using multi-path particle dosimetry model. Toxicol. Res. 1, 206–210. https://doi.org/10.1039/C2TX20029E (2012).
Choi, Y., Choi, I. W. & Kwon, J-H. Assessing inhalation intake of microplastics using MPPD model. Environ. Anal. Health Toxicol. 40, 2025s02. https://doi.org/10.5620/eaht.2025s02 (2025).
Jabbal, S., Poli, G. & Lipworth, B. Does size really matter? Relationship of particle size to lung deposition and exhaled fraction. J. Allergy Clin. Immun. 139, 2013–2014. https://doi.org/10.1016/j.jaci.2016.11.036 (2017).
Voutilainen, A., Kaipio, J. P., Pekkanen, J., Timonen, K. L. & Ruuskanen, J. Theoretical analysis of the influence of aerosol size distribution and physical activity on particle deposition pattern in human lungs. Scand. J. Work Environ. Health. 30, 73–79 (2004).
Kim, J., Jo, G., Kim, H. & Park, D. A review on the health risks associated with the use of products containing Benzalkonium chloride (BKC), focusing on humidifier disinfectant products. J. Environ. Health Sci. 47, 513–520. https://doi.org/10.5668/JEHS.2021.47.6.513 (2021). (In Korean).
OECD. Guidance Document on Inhalation Toxicity Studies (OECD Publishing, 2018).
Amin, F., Roohbakhsh, A., Memarzia, A., Kazerani, H. R. & Boskabady, M. H. Immediate and late systemic and lung effects of inhaled Paraquat in rats. J. Hazard. Mater. 415, 125633. https://doi.org/10.1016/j.jhazmat.2021.125633 (2021).
Choi, H-Y. et al. Assessment of respiratory and systemic toxicity of benzalkonium chloride following a 14-day inhalation study in rats. Part. Fibre Toxicol. 2020;17:1–19. (2020). https://doi.org/10.1186/s12989-020-0339-8
Ghasemi, S. Z. et al. Evaluation of nano-curcumin against inhaled paraquat-induced lung injury in rats. Pharmacol. Rep. 75, 671–681. https://doi.org/10.1007/s43440-023-00483-3 (2023).
KEITI. Development of Risk Assessment Method of Biocide Active Ingredients. (2015).
Kim, Y-S., Lee, S-B. & Lim, C-H. Effects of Didecyldimethylammonium chloride (DDAC) on Sprague-Dawley rats after 13 weeks of inhalation exposure. Toxicol. Res. 33, 7–14. https://doi.org/10.5487/TR.2017.33.1.007 (2017).
KOSHA. Benzalkonium chloride – Acute inhalation toxicity report in rat. file:///C:/Users/user/Downloads/%EA%B8%89%EC%84%B136_2018_Benzalkonium_chloride_Rat%20(1).pdf
KOSHA. Benzalkonium chloride – Subchronic inhalation toxicity report in rat. file:///C:/Users/user/Downloads/%EB%B0%98%EB%B3%B546_2019_Benzalkonium_chloride_90day_Rat%20(2).pdf
Lim, C-H. & Chung, Y-H. Effects of Didecyldimethylammonium chloride on sprague-dawley rats after two weeks of inhalation exposure. Toxicol. Res. 30, 205–210. https://doi.org/10.5487/TR.2014.30.3.20 (2014).
NIER. A safety study for management of the existing chemicals (I). (2013).
Bermudez, E. et al. Pulmonary responses of mice, rats, and hamsters to subchronic inhalation of ultrafine titanium dioxide particles. Toxicol. Sci. 77, 347–357. https://doi.org/10.1093/toxsci/kfh019 (2004).
Miller, F. J. et al. The fractions of respiratory tract cells at risk in formaldehyde carcinogenesis. Inhal Toxicol. 23, 689–706. https://doi.org/10.3109/08958378.2011.603190 (2011).
Park, D-U. et al. Properties of polyhexamethylene guanidine (PHMG) associated with fatal lung injury in Korea. Molecules 25, 3301. https://doi.org/10.3390/molecules25143301 (2020).
Hofmann, W. Modelling inhaled particle deposition in the human lung—A review. J. Aerosol Sci. 42, 693–724. https://doi.org/10.1016/j.jaerosci.2011.05.007 (2011).
Green, H. L. & Lane, W. R. Particulate Clouds: dusts, Smokes and Mists: their Physics and Physical Chemistry and Industrial and Environmental Aspects 2nd edn (E.&F.N. Spon. Ltd., 1964).
Nunes, C. et al. An in vitro strategy using multiple human induced pluripotent stem cell-derived models to assess the toxicity of chemicals: A case study on Paraquat. Toxicol. Vitro. 81, 105333. https://doi.org/10.1016/j.tiv.2022.105333 (2022).
Funding
This work was supported by the Korea Environment Industry & Technology Institute (KEITI) through the Technology Development Project for Safety Management of Household Chemical Products Program funded by the Korea Ministry of Environment (MOE) (202300230430).
Author information
Authors and Affiliations
Contributions
YC: Conceptualisation, Methodology, Investigation, Data curation, Writing – Original draft preparation; HRK: Writing – Review & editing, Funding acquisition; JHK: Conceptualisation, Methodology, Data curation, Writing – Review & editing, Supervision, Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study did not involve any experiments utilizing live animals. All investigations were conducted using a laboratory test chamber without animals. Therefore, the study did not require ethical approval for animal experiments. All comparative data derived from external sources are appropriately cited in the main body or supplementary information.
Consent for publication
All authors have seen and approved the final version of the manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Choi, Y., Kim, H.R. & Kwon, JH. Concentration-dependent aerosol size alters regional deposition and inhalation dose translation. Sci Rep (2026). https://doi.org/10.1038/s41598-026-35566-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-35566-7