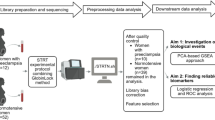
Abstract
Lymphatic vasculature regulates lymphocyte trafficking and modulates adaptive immunity. Imbalanced immune cells at the maternal-fetal interface may contribute to severe preeclampsia (PE). Impaired placental lymphangiogenesis and immune dysregulation could contribute to PE but supporting evidence is limited. Here, we investigate the association between lymphangiogenesis and immune regulation in severe PE. First, we identified the presence of LYVE1-positive lymphatic vessels in the decidua, and then decidual lymphatic endothelial cells (dLECs) were isolated and cultured from chorioamniotic membranes obtained at cesarean section from women with PE (n = 15) and gestational age-matched controls (n = 15). The cells were identified by LYVE1, Prox1, and CD31 expression. Gene expression analysis showed the significant different gene expression profiles in PE compared to normal (lymphatic vessel development, immune cell trafficking and T-cell activation regulation). dLECs from PE pregnancies showed substantially reduced migration, adhesion, morphological differentiation, and decreased lymphatic sprouting in a 3D lymphatic ring assay compared with normal. Additionally, they exhibited low chemokine ligand 21 expression, impaired dendritic cell recruitment, and reduced Akt-eNOS-nitric oxide signaling, which suppresses decidual cytotoxic T-cell activation in decidua. Collectively, our findings suggest that impaired lymphatic vessel function and molecular alterations in the decidua may disrupt immune regulation and contribute to severe PE.
Similar content being viewed by others
Data availability
The datasets generated and/or analysed during the current study are available in the NCBI repository, [BioProject ID: PRJNA1345170]
References
Duley, L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 33, 130–137 (2009).
Ananth, C. V., Keyes, K. M. & Wapner, R. J. Pre-eclampsia rates in the united States, 1980–2010: age-period-cohort analysis. BMJ 347, 6564 (2013).
Pratt, A. et al. Placenta-derived angiogenic proteins and their contribution to the pathogenesis of preeclampsia. Angiogenesis 18, 115–123 (2015).
Possomato-Vieira, J. S. & Khalil, R. A. Mechanisms of endothelial dysfunction in hypertensive pregnancy and preeclampsia. Adv. Pharmacol. 77, 361–431 (2016).
Brosens, I. Spiraled arterioles of the decidua basalis in the hypertensive complications of pregnancy. Anatomoclinical study. Bull. Soc. R Belge Gynecol. Obstet. 33, 61–72 (1963).
Brosens, I. A study of the spiral arteries of the decidua basalis in normotensive and hypertensive pregnancies. J. Obstet. Gynaecol. Br. Commonw. 71, 222–230 (1964).
Espinoza, J. et al. Normal and abnormal transformation of the spiral arteries during pregnancy. J. Perinat. Med. 34, 447–458 (2006).
Labarrere, C. A. et al. Failure of physiologic transformation of spiral arteries, endothelial and trophoblast cell activation, and acute atherosis in the basal plate of the placenta. Am. J. Obstet. Gynecol. 216, 287.e1-287.e16 (2017).
Moffett, A. & Loke, C. Immunology of placentation in eutherian mammals. Nat. Rev. Immunol. 6, 584–594 (2006).
Leslie, M. & Immunology Fetal immune system hushes attacks on maternal cells. Science 322, 1450–1451 (2008).
Burlingham, W. J. A lesson in tolerance – maternal instruction to fetal cells. N Engl. J. Med. 360, 1355–1357 (2009).
Valencia-Ortega, J., Saucedo, R., Peña‐Cano, M. I., Hernández‐Valencia, M. & Cruz‐Durán, J. G. Immune tolerance at the maternal‐placental interface in healthy pregnancy and pre‐eclampsia. J. Obstet. Gynaecol. Res. 46, 1067–1076 (2020).
Deer, E. et al. The role of immune cells and mediators in preeclampsia. Nat. Rev. Nephrol. 19, 257–270 (2023).
Joo, J. S., Lee, D. & Hong, J. Y. Multi-layered mechanisms of immunological tolerance at the maternal-fetal interface. Immune Netw. 24 (4), e30 (2024).
Kumar, V. & Stewart, J. H. 4 The complement system in human pregnancy and preeclampsia. Front. Immunol. 16, 1617140 (2025).
Jung, Y. J. et al. Abnormal lymphatic vessel development is associated with decreased decidual regulatory T cells in severe preeclampsia. Am. J. Reprod. Immunol. 80, e12970 (2018).
Leber, A., Teles, A. & Zenclussen, A. C. Regulatory T cells and their role in pregnancy. Am. J. Reprod. Immunol. 63, 445–459 (2010).
Zenclussen, A. C. Regulatory T cells in pregnancy. Springer Semin Immunopathol. 28, 31–39 (2006).
Saito, S., Shiozaki, A., Nakashima, A., Sakai, M. & Sasaki, Y. The role of the immune system in preeclampsia. Mol. Aspects Med. 28, 192–209 (2007).
Tsuda, S., Nakashima, A., Shima, T. & Saito, S. New paradigm in the role of regulatory T cells during pregnancy. Front. Immunol. 10, 573 (2019).
Saito, S., Sasaki, Y. & Sakai, M. CD4(+)CD25high regulatory T cells in human pregnancy. J. Reprod. Immunol. 65, 111–120 (2005).
Red-Horse, K. Lymphatic vessel dynamics in the uterine wall. Placenta 29, S55–S59 (2008).
Jung, Y. J. et al. Decidual lymphatic endothelial cell-derived granulocyte‐macrophage colony‐stimulating factor induces M1 macrophage polarization via the NF‐κB pathway in severe pre‐eclampsia. Am. J. Reprod. Immunol. 90, e13744 (2023).
Volchek, M. et al. Lymphatics in the human endometrium disappear during decidualization. Hum. Reprod. 25, 2455–2464 (2010).
Red-Horse, K. et al. Cytotrophoblast induction of arterial apoptosis and lymphangiogenesis in an in vivo model of human placentation. J. Clin. Invest. 116, 2643–2652 (2006).
Zhou, Y. et al. Cytotrophoblast induction of arterial apoptosis and lymphangiogenesis in an in vivo model of human placentation. J. Clin. Invest. 116, 2643–2652 (2006).
Ji, L. et al. The defect of both angiogenesis and lymphangiogenesis is involved in preeclampsia. Placenta 36, 279–286 (2015).
Lucas, E. D. & Tamburini, B. A. J. Lymph node lymphatic endothelial cell expansion and contraction and the programming of the immune response. Front. Immunol. 10, 36 (2019).
Jalkanen, S. & Salmi, M. Lymphatic endothelial cells of the lymph node. Nat. Rev. Immunol. 20, 566–578 (2020).
Tewalt, E. F., Cohen, J. N., Rouhani, S. J. & Engelhard, V. H. Lymphatic endothelial cells–key players in regulation of tolerance and immunity. Front. Immunol. 3, 305 (2012).
Cifarelli, V. et al. Visceral obesity and insulin resistance associate with CD36 deletion in lymphatic endothelial cells. Nat. Commun. 12, 3350 (2021).
Simeroth, S. & Yu, P. The role of lymphatic endothelial cell metabolism in lymphangiogenesis and disease. Front. Cardiovasc. Med. 11, 1392816 (2024).
Harlé, G. et al. Macroautophagy in lymphatic endothelial cells inhibits T cell–mediated autoimmunity. J. Exp. Med. 218, e20201776 (2021).
Wang, Y., Li, B. & Zhao, Y. Inflammation in preeclampsia: genetic biomarkers, mechanisms and therapeutic strategies. Front. Immunol. 13, 883404 (2022).
Torres-Torres, J. et al. A narrative review on the pathophysiology of preeclampsia. Int. J. Mol. Sci. 25, 7569 (2024).
Dimitriadis, E. et al. Pre-eclampsia. Nat. Rev. Dis. Primers. 9, 8 (2023).
Johnson, L. A. & Jackson, D. G. Inflammation-induced secretion of CCL21 in lymphatic endothelium is a key regulator of integrin-mediated dendritic cell transmigration. Int. Immunol. 22, 839–849 (2010).
Gunn, M. D. et al. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 95, 258–263 (1998).
Kriehuber, E. et al. Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J. Exp. Med. 194, 797–808 (2001).
Cyr, A. R., Huckaby, L. V., Shiva, S. S. & Zuckerbraun, B. S. Nitric oxide and endothelial dysfunction. Crit. Care Clin. 36, 307–321 (2020).
Hagendoorn, J. et al. Endothelial nitric oxide synthase regulates microlymphatic flow via collecting lymphatics. Circ. Res. 95, 204–209 (2004).
Lukacs-Kornek, V. et al. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat. Immunol. 12, 1096–1104 (2011).
Natarajan, M. et al. Inhibitor-κB kinase attenuates Hsp90-dependent endothelial nitric oxide synthase function in vascular endothelial cells. Am. J. Physiol. Cell. Physiol. 308, C673–C683 (2015).
Coultrap, S. J. & Bayer, K. U. Nitric oxide induces Ca2+-independent activity of the Ca2+/calmodulin-dependent protein kinase II (CaMKII). J. Biol. Chem. 289, 19458–19465 (2014).
Jung, E. et al. The etiology of preeclampsia. Am. J. Obstet. Gynecol. 226, S844–S866 (2022).
Redman, C. W. G. & Sargent, I. L. Immunology of pre-eclampsia. Am. J. Reprod. Immunol. 63, 534–543 (2010).
Reyes, L. & Golos, T. G. Hofbauer cells: their role in healthy and complicated pregnancy. Front. Immunol. 9, 2628 (2018).
Reister, F. et al. The distribution of macrophages in spiral arteries of the placental bed in pre-eclampsia differs from that in healthy patients. Placenta 20, 229–233 (1999).
Prins, J. R. et al. Preeclampsia is associated with lower percentages of regulatory T cells in maternal blood. Hypertens. Pregnancy. 28, 300–311 (2009).
Ribeiro, V. R. et al. Association between cytokine profile and transcription factors produced by T-cell subsets in early‐ and late‐onset pre‐eclampsia. Immunology 152, 163–173 (2017).
Liu, H. et al. The defect of both angiogenesis and lymphangiogenesis is involved in preeclampsia. Placenta 36, 279–286 (2015).
Rutkowski, J. M. et al. VEGFR-3 neutralization inhibits ovarian lymphangiogenesis, follicle maturation, and murine pregnancy. Am. J. Pathol. 183, 1596–1607 (2013).
Fristiohady, A. et al. 12(S)-HETE induces lymph endothelial cell Retraction in vitro by upregulation of SOX18. Int. J. Oncol. 53, 307–316 (2018).
François, M. et al. Sox18 induces development of the lymphatic vasculature in mice. Nature 456, 643–647 (2008).
Srinivasan, R. S. et al. The nuclear hormone receptor Coup-TFII is required for the initiation and early maintenance of Prox1 expression in lymphatic endothelial cells. Genes Dev. 24, 696–707 (2010).
Zhu, L. et al. PROX1 promotes breast cancer invasion and metastasis through WNT/β-catenin pathway via interacting with HnRNPK. Int. J. Biol. Sci. 18, 2032–2046 (2022).
Banerji, S. et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell. Biol. 144, 789–801 (1999).
Jackson, D. G. Lymphatic trafficking of immune cells and insights for cancer metastasis. Clin. Exp. Metastasis. 41, 381–386 (2024).
Kruger, R. P., Aurandt, J. & Guan, K. L. Semaphorins command cells to move. Nat. Rev. Mol. Cell. Biol. 6, 789–800 (2005).
Sakurai, A., Doçi, C. L. & Gutkind, J. S. Semaphorin signaling in angiogenesis, lymphangiogenesis and cancer. Cell. Res. 22, 23–32 (2012).
Kiseleva, E. P. & Rutto, K. V. Semaphorin 3A in the immune system: Twenty years of study. Biochem. (Mosc). 87, 640–657 (2022).
Ricci, M. et al. Review of the function of SEMA3A in lymphatic vessel maturation and its potential as a candidate gene for lymphedema: analysis of three families with rare causative variants. Lymphology 53, 63–75 (2020).
Bouvrée, K. et al. Semaphorin3A, neuropilin-1, and PlexinA1 are required for lymphatic valve formation. Circ. Res. 111, 437–445 (2012).
Russo, E., Nitschké, M. & Halin, C. Dendritic cell interactions with lymphatic endothelium. Lymphat Res. Biol. 11, 172–182 (2013).
Martín-Fontecha, A. et al. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J. Exp. Med. 198, 615–621 (2003).
Randolph, G. J., Angeli, V. & Swartz, M. A. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 5, 617–628 (2005).
Tal, O. et al. DC mobilization from the skin requires Docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. J. Exp. Med. 208, 2141–2153 (2011).
Mori, S. et al. Mice lacking expression of the chemokines CCL21-ser and CCL19 (plt mice) demonstrate delayed but enhanced T cell immune responses. J. Exp. Med. 193, 207–218 (2001).
Ou, J., Ou, Z., Ackerman, A. W., Oldham, K. T. & Pritchard, K. A. Jr. Inhibition of heat shock protein 90 (hsp90) in proliferating endothelial cells uncouples endothelial nitric oxide synthase activity. Free Radic Biol. Med. 34, 269–276 (2003).
Pritchard, K. A. et al. Heat shock protein 90 mediates the balance of nitric oxide and superoxide anion from endothelial nitric-oxide synthase. J. Biol. Chem. 276, 17621–17624 (2001).
Prangsaengtong, O., Koizumi, K., Senda, K., Sakurai, H. & Saiki, I. ENOS and Hsp90 interaction directly correlates with cord formation in human lymphatic endothelial cells. Lymphat Res. Biol. 9, 53–59 (2011).
Prangsaengtong, O. et al. Calpain 1 and – 2 play opposite roles in cord formation of lymphatic endothelial cells via eNOS regulation. Hum. Cell. 25, 36–44 (2012).
Sumi, M. P. & Ghosh, A. Hsp90 in human diseases: molecular mechanisms to therapeutic approaches. Cells 11, 976 (2022).
Scarneo, S. A. et al. Expression of membrane Hsp90 is a molecular signature of T cell activation. Sci. Rep. 12, 18091 (2022).
Förstermann, U. & Sessa, W. C. Nitric oxide synthases: regulation and function. Eur. Heart J. 33, 829–837 (2012).
Cho, H. J. et al. Calmodulin is a subunit of nitric oxide synthase from macrophages. J. Exp. Med. 176, 599–604 (1992).
Niedbala, W. et al. Nitric oxide enhances Th9 cell differentiation and airway inflammation. Nat. Commun. 5, 4575 (2014).
Yang, J. et al. T cell–derived inducible nitric oxide synthase switches off Th17 cell differentiation. J. Exp. Med. 210, 1447–1462 (2013).
Niedbala, W. et al. Nitric oxide–induced regulatory T cells inhibit Th17 but not Th1 cell differentiation and function. J. Immunol. 191, 164–170 (2013).
Ibiza, S. et al. Endothelial nitric oxide synthase regulates T cell receptor signaling at the immunological synapse. Immunity 24, 753–765 (2006).
Ibiza, S. et al. Endothelial nitric oxide synthase regulates N-Ras activation on the golgi complex of antigen-stimulated T cells. Proc. Natl. Acad. Sci. U S A. 105, 10507–10512 (2008).
Nagy, G., Koncz, A. & Perl, A. T cell activation-induced mitochondrial hyperpolarization is mediated by Ca2+- and redox-dependent production of nitric oxide. J. Immunol. 171, 5188–5197 (2003).
Blesson, S. et al. Analysis of the mechanisms of human cytotoxic T lymphocyte response Inhibition by NO. Int. Immunol. 14, 1169–1178 (2002).
Wang, Y. Y., Zhao, R. & Zhe, H. The emerging role of camkii in cancer. Oncotarget 6, 11725–11734 (2015).
Liu, Q. et al. Wnt5a/CaMKII/ERK/CCL2 axis is required for tumor-associated macrophages to promote colorectal cancer progression. Int. J. Biol. Sci. 16, 1023–1034 (2020).
Guerin, L. R., Prins, J. R. & Robertson, S. A. Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum. Reprod. Update. 15 (5), 517–535 (2009).
de Alwis, N. et al. Novel approaches to combat preeclampsia: from new drugs to innovative delivery. Placenta 102, 10–16 (2020).
Hypertension in pregnancy. Report of the American college of obstetricians and gynecologists’ task force on hypertension in pregnancy. Obstet. Gynecol. 122, 1122–1131 (2013).
Aguilar, B. et al. Lymphatic reprogramming by Kaposi sarcoma herpes virus promotes the oncogenic activity of the virus-encoded G-protein-coupled receptor. Cancer Res. 72, 5833–5842 (2012).
Meyer, T. P. et al. Filter Buffy coats (FBC): a source of peripheral blood leukocytes recovered from leukocyte depletion filters. J. Immunol. Methods. 307, 150–166 (2005).
Rouzaut, A. et al. Dendritic cells adhere to and transmigrate across lymphatic endothelium in response to IFN-α. Eur. J. Immunol. 40, 3054–3063 (2010).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with bowtie 2. Nat. Methods. 9, 357–359 (2012).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
R Core Team. R: a language and environment for statistical computing. https://www.r-project.org/ (R Foundation for Statistical Computing, 2020).
Gentleman, R. C. et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5, R80 (2004).
Praveen Kumar, S. T. P. & Aswath, N. DNA isolation from teeth by organic extraction and identification of sex of the individual by analyzing the AMEL gene marker using PCR. J. Forensic Dent. Sci. 8, 18–21 (2016).
Butler, E. & Li, R. Genetic markers for sex identification in forensic DNA analysis. J. Forensic Investig. 2, 10 (2014).
Kwon, H., Kwon, J. Y., Song, J. & Maeng, Y. S. Decreased lymphangiogenic activities and genes expression of cord blood lymphatic endothelial progenitor cells (Vegfr3+/pod+/cd11b + cells) in patient with preeclampsia. Int. J. Mol. Sci. 22, 4237 (2021).
Joshi, M. S. et al. Receptor-mediated activation of nitric oxide synthesis by arginine in endothelial cells. Proc. Natl. Acad. Sci. U. S. A. 104, 9982–9987 (2007).
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2021R1A2C2014591, 2022R1I1A1A01064011, and RS-2024-00342487).
Author information
Authors and Affiliations
Contributions
S.K., Y. L., and Y.M. designed the study and performed the experiments. S.K., J.K., and Y.M. contributed to the interpretation of the results. S.K., J.K., and Y.M. wrote the article. All authors provided critical feedback and helped shape the research, analysis, and manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics declarations
The study had been approved by the Institutional Review Board of Severance Hospital (4-2016-0450). Informed consent was obtained from all study participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, S., Lee, Y., Kwon, JY. et al. Immune regulation and lymphangiogenesis by lymphatic endothelial cells in the decidua in severe preeclampsia. Sci Rep (2026). https://doi.org/10.1038/s41598-026-35667-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-35667-3