Abstract
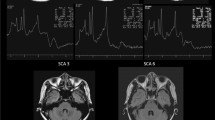
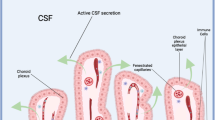
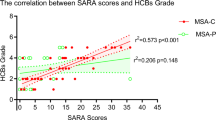
The choroid plexus (CP), a component of the glymphatic system, is essential in homeostasis and producing cerebrospinal fluid. Role of CP in multiple system atrophy (MSA) remains unclear. This study aimed to investigate the implication of the CP in MSA. This retrospective cross-sectional study included 87 MSA patients who underwent the Unified MSA Rating Scale (UMSARS), brain MRI, and18F-fluorodeoxyglucose PET scan, along with 84 healthy controls (HCs). Multivariate linear regression analyses were performed to examine the associations between CP volume (CPV) and UMSARS scores, as well as the volumes and cerebral metabolism. Compared with HCs, MSA had significantly reduced CPV (1.00 ± 0.27 vs. 1.30 ± 0.26, P < 0.001). CPV showed no association with UMSARS, however, it was positively correlated with regional cerebellar volumes. Reduced CPV was associated with lower cerebral glucose metabolism in MSA-susceptible regions, consistent with the positive association between CPV and regional cerebral glucose metabolism observed in multivariate analyses. Notably, CPV positively correlated with glucose metabolism in the brainstem (β = 0.110, P = 0.003) and cerebellar white matter (β = 0.080, P = 0.004). This study suggests that CPV is positively associated with disease burden in MSA, with CPV decreasing as disease severity increases. Further research is warranted to determine whether CPV could serve as a potential biomarker for MSA.
Similar content being viewed by others
Data availability
Data generated or analyzed during the study are available from the corresponding author by request.
References
Kratzer, I., Ek, J. & Stolp, H. The molecular anatomy and functions of the choroid plexus in healthy and diseased brain. Biochim. Biophys. Acta Biomembr. 1862, 183430. https://doi.org/10.1016/j.bbamem.2020.183430 (2020).
Mogensen, F. L., Delle, C. & Nedergaard, M. The glymphatic system (En)during inflammation. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22147491 (2021).
Harrison, I. F. et al. Impaired glymphatic function and clearance of Tau in an Alzheimer’s disease model. Brain 143, 2576–2593. https://doi.org/10.1093/brain/awaa179 (2020).
Ricigliano, V. A. G. et al. Choroid plexus enlargement in inflammatory multiple sclerosis: 3.0-T MRI and translocator protein PET evaluation. Radiology 301, 166–177. https://doi.org/10.1148/radiol.2021204426 (2021).
Liu, R. et al. Choroid plexus epithelium and its role in neurological diseases. Front. Mol. Neurosci. 15, 949231. https://doi.org/10.3389/fnmol.2022.949231 (2022).
Tadayon, E., Pascual-Leone, A., Press, D. & Santarnecchi, E. Choroid plexus volume is associated with levels of CSF proteins: relevance for Alzheimer’s and Parkinson’s disease. Neurobiol. Aging. 89, 108–117. https://doi.org/10.1016/j.neurobiolaging.2020.01.005 (2020).
Tadayon, E. et al. Improving choroid plexus segmentation in the healthy and diseased brain: relevance for Tau-PET imaging in dementia. J. Alzheimers Dis. 74, 1057–1068. https://doi.org/10.3233/jad-190706 (2020).
Dai, T. et al. Choroid plexus enlargement in amyotrophic lateral sclerosis patients and its correlation with clinical disability and blood-CSF barrier permeability. Fluids Barriers CNS. 21, 36. https://doi.org/10.1186/s12987-024-00536-6 (2024).
Liu, L. et al. Choroid plexus enlargement contributes to motor severity via regional glymphatic dysfunction in Parkinson’s disease. NPJ Parkinsons Dis. 11, 134. https://doi.org/10.1038/s41531-025-00971-8 (2025).
Jeong, S. H. et al. Association between choroid plexus volume and cognition in Parkinson disease. Eur. J. Neurol. 30, 3114–3123. https://doi.org/10.1111/ene.15999 (2023).
Shi, C. et al. Impaired glymphatic clearance in multiple system atrophy: A diffusion spectrum imaging study. Parkinsonism Relat. Disord. 123, 106950. https://doi.org/10.1016/j.parkreldis.2024.106950 (2024).
Jeong, S. H. et al. Choroid plexus Volume, amyloid Burden, and cognition in the Alzheimer’s disease continuum. Aging Dis. 16, 552–564. https://doi.org/10.14336/ad.2024.0118 (2024).
Assogna, M. et al. Association of choroid plexus volume with serum biomarkers, clinical features, and disease severity in patients with frontotemporal Lobar degeneration spectrum. Neurology 101, e1218–e1230. https://doi.org/10.1212/wnl.0000000000207600 (2023).
Wang, N. et al. Choroid plexus enlargement correlates with subcortical Tau deposition in progressive supranuclear palsy. Fluids Barriers CNS. 22, 52. https://doi.org/10.1186/s12987-025-00663-8 (2025).
Li, J. et al. Associations between the choroid plexus and Tau in Alzheimer’s disease using an active learning segmentation pipeline. Fluids Barriers CNS. 21, 56. https://doi.org/10.1186/s12987-024-00554-4 (2024).
Shi, C. et al. Impaired glymphatic clearance in multiple system atrophy: A diffusion spectrum imaging study. Parkinsonism Relat. Disord. 123, 106950. https://doi.org/10.1016/j.parkreldis.2024.106950 (2024).
Municio, C., Carrero, L., Antequera, D. & Carro, E. Choroid plexus aquaporins in CSF homeostasis and the glymphatic system: Their relevance for Alzheimer’s disease. Int. J. Mol. Sci. https://doi.org/10.3390/ijms24010878 (2023).
Braun, M. & Iliff, J. J. The impact of neurovascular, blood-brain barrier, and glymphatic dysfunction in neurodegenerative and metabolic diseases. Int. Rev. Neurobiol. 154, 413–436. https://doi.org/10.1016/bs.irn.2020.02.006 (2020).
Jessen, N. A., Munk, A. S., Lundgaard, I. & Nedergaard, M. The glymphatic system: A beginner’s guide. Neurochem. Res. 40, 2583–2599. https://doi.org/10.1007/s11064-015-1581-6 (2015).
Palma, J. A. et al. Limitations of the unified multiple system atrophy rating scale as outcome measure for clinical trials and a roadmap for improvement. Clin. Auton. Res. 31, 157–164. https://doi.org/10.1007/s10286-021-00782-w (2021).
Chelban, V. et al. An update on advances in magnetic resonance imaging of multiple system atrophy. J. Neurol. 266, 1036–1045. https://doi.org/10.1007/s00415-018-9121-3 (2019).
Tsitsou-Kampeli, A., Suzzi, S. & Schwartz, M. The immune and metabolic milieu of the choroid plexus as a potential target in brain protection. Trends Neurosci. 47, 573–582. https://doi.org/10.1016/j.tins.2024.05.010 (2024).
Park, K. W. et al. Cortical hypometabolism associated with cognitive impairment of multiple system atrophy. Parkinsonism Relat. Disord. 81, 151–156. https://doi.org/10.1016/j.parkreldis.2020.10.039 (2020).
Jiang, J. et al. Study of the influence of age in (18)F-FDG PET images using a data-driven approach and its evaluation in Alzheimer’s disease. Contrast Media Mol. Imaging 2018, 3786083. https://doi.org/10.1155/2018/3786083 (2018).
Ding, X. B. et al. Impaired meningeal lymphatic drainage in patients with idiopathic parkinson’s disease. Nat. Med. 27, 411–418. https://doi.org/10.1038/s41591-020-01198-1 (2021).
Serot, J. M., Béné, M. C., Foliguet, B. & Faure, G. C. Morphological alterations of the choroid plexus in late-onset Alzheimer’s disease. Acta Neuropathol. 99, 105–108. https://doi.org/10.1007/pl00007412 (2000).
Wenning, G. K. et al. The movement disorder society criteria for the diagnosis of multiple system atrophy. Mov. Disord. 37, 1131–1148. https://doi.org/10.1002/mds.29005 (2022).
Henschel, L. et al. FastSurfer—A fast and accurate deep learning based neuroimaging pipeline. Neuroimage 219, 117012. https://doi.org/10.1016/j.neuroimage.2020.117012 (2020).
Acknowledgements
We acknowledge the Korea government (MSIT) for assistance and support.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. RS-2023-00209580).
Author information
Authors and Affiliations
Contributions
C.J.P., Y.S., S.H.J., and P.H.L. conceptualized and designed the study. Y.S., H.K.N., S.K.L., Y.H.S., and P.H.L contributed to the acquisition of the data. C.J.P., Y.S., H.J.J., C.H.L., and S.H.J. contribute to analysis of the data. C.J.P., Y.S., S.H.J., and P.H.L. drafted and/or revised the manuscript. S.H.J., and P.H.L. are the guarantor.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Park, C.J., Sun, Y., Jeong, HJ. et al. Association of choroid plexus volume with brain atrophy and glucose metabolism in multiple system atrophy. Sci Rep (2026). https://doi.org/10.1038/s41598-026-35850-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-35850-6