Abstract
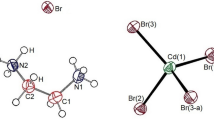
Single crystals of the organic–inorganic hybrid compound [N(C3H7)4]2Cd2Cl6 were synthesized by an aqueous solution method, and their structural and dynamic properties were systematically investigated. Thermal analyses revealed phase transitions at 321 K (TC1) and 445 K (TC2), followed by melting at 476 K. Single-crystal X-ray diffraction indicated that the compound crystallizes in a triclinic system with space group P\(\:\stackrel{-}{1}\), showing variations in lattice parameters across TC1 while maintaining the same symmetry. On the other hand, whereas the 1H, 13C, and 14N MAS NMR chemical shifts of the [N(C3H7)4] cations showed little variation near TC1, the 113Cd MAS NMR spectra of the Cd2Cl6 anions revealed a clear change, with two Cd sites (Cd(1) and Cd(2)) merging into a single site. This agrees well with the SCXRD results, indicating that the phase transition at TC1 arises primarily from an order–disorder transition of the Cd2Cl6 anions rather than the [N(C3H7)4] cations. In addition, temperature-dependent line narrowing in the1H, 13C, 14N, and 113Cd NMR spectra reflects enhanced molecular dynamics at elevated temperatures. These results provide insights into the phase behavior and stability of [N(C3H7)4]2Cd2Cl6, highlighting its potential relevance for future functional applications.
Similar content being viewed by others
Data availability
The datasets generated and/or analysed during the current study are available in the CCDC 2491598, 2491602.
References
Rao, C. N. R., Cheetham, A. K. & Thirumurugan, A. Hybrid inorganic–organic materials: a new family in condensed matter physics. J. Phys. : Condens. Matter. 20, 083202 (2008).
Shao, T. et al. F. 2D lead-free organic–inorganic hybrid exhibiting dielectric and structural phase transition at higher temperatures. Cryst. Eng. Comm. 24, 4346 (2022).
Han, K. et al. Organic–inorganic hybrid compound [H2-1,5-Diazabicyclo[3.3.0]octane]ZnBr4 with reverse symmetry breaking shows a switchable dielectric anomaly and robust second harmonic generation effect. Inorg. Chem. 61, 11859 (2022).
Saikumar, I., Ahmad, S., Baumberg, J. J. & Prakash, V. Fabrication of excitonic luminescent inorganic–organic hybrid nano- and microcrystals. Scr. Mater. 67, 834 (2012).
Staskiewicz, B., Czupinski, O. & Czapla, Z. On some spectroscopic properties of a layered 1,3-diammoniumpropylene tetrabromocadmate hybrid crystal. J. Mol. Struct. 1074, 723 (2014).
Lim, A. R. Crystal structure, structural geometry, and molecular motion of organic-inorganic perovskite [N(CH3)4]2MnCl4 crystals at phases I, II, and III. Sci. Rep. 14, 26607 (2024).
Na, C. & Lim, A. R. Elucidation of crystal growth, structural characterization, thermal properties, and molecular dynamics using NMR near phase transition temperature of [N(CH3)4]2CoCl4. Sci. Rep. 15, 28107 (2025).
El-Korsahy, A. & Brik, M. G. Crystal growth, spectroscopic and crystal field studies of [N(CH3)4]2MnCl4 and [N(CH3)4]2CoCl4 single crystals in the paraelectric phase. Solid State Commun. 135, 298 (2005).
Batyuk, A. Y., Kapustyanyk, V. B. & Korchak, Y. M. Manifestations of phase transitions and the thermooptical-memory effect in the absorption spectra of (N(CH3)4)2CuCl4 crystals. J. Appl. Spectrosc. 72, 413 (2005).
Abu El-Fadl, A., El-Korashy, A. & El-Zahed, H. Electrical investigations in the normal and incommensurate phases of [N(CH3)4]2ZnCl4 single crystals. J. Phys. Chem. Solids. 63, 375 (2002).
Koshiji, N., Miyoshi, T. & Mashiyama, H. Electron density distribution of the commensurate phase of TMATC-Mn. Ferroelectrics 440, 119 (2012).
Koshiji, N. & Mashiyama, H. Structural study of ordering in the normal-commensurate transition of {N(CH3)4}2MnCl4. J. Phys. Soc. Japan. 80, 64602 (2011).
Brik, M. G., El-Korashy, A. & Almokhtar, M. Exchange charge model calculations of crystal field parameters and crystal field energy levels for [N(CH3)4]2CoCl4 and [N(CH3)4]2MnCl4 single crystals. J. Alloys Compd. 459, 71 (2008).
Koshiji, N. & Mashiyama, H. Disordered and displacive models for the structure of the normal phase in {N(CH3)4}2MnCl4. J. Phys. Soc. Japan. 69, 3853 (2000).
Koksal, F., Bahadir, S., Basaran, E. & Yerli, Y. Temperature independent isotropic EPR spectra of [(CH3)4N]2MnCl4 and [(CH3)4N]2FeCl4 single crystals. Z. Naturforsch. 54a, 557 (1999).
El-Korashy, A., El-Zahed, H. & Radwan, M. Optical studies of [N(CH3)4]2CoCl4, [N(CH3)4]2MnCl4 single crystals in the normal paraelectric phase. Physica B. 334, 75 (2003).
Bolesta, I., Furgala, Y. & Kityk, I. Effects of phase transitions in luminescience features of [N(CH3)4]2MnCl4 single crystals. Ferroelectrics 56, 1 (1996).
Marco de Lucas, M. C., Rodriguez, F. & Moreno, M. Optical investigations on {N(CH3)4}2MnCl4: a new phase transition at 90 K. Ferroelectrics 109, 21 (1990).
Fuith, A., Schranz, W., Warhanek, H., Kroupa, J. & Lhotska, V. Optical investigations in [N(CH3)4]2MnCl4. Phase Transitions. 27, 15 (1990).
Shimomura, S., Hamaya, N. & Fujii, Y. High-resolution X-ray diffraction study of commensurate-incommensurate phase transition in [N(CH3)4]2MnCl4 under pressure. J. Phys. Soc. Japan. 65, 661 (1996).
Shimomura, S., Hamaya, N. & Fujii, Y. Systematics in the commensurate-incommensurate phase transition in [N(CH3)4]2MCl4 (M = Zn, Fe, and Mn) under pressure. Phys Rev. B. 53, 8975 (1996).
Hamaya, N., Shimomura, S. & Fujii, Y. Systematics in the modulated phases of [N(CH3)4]2MnCl4. J. Phys. : Condens. Matter. 3, 3387 (1991).
Karpa, I. V. et al. Electronic spectra of [N(CH3)4]2CoCl4 microcrystals in thin films. J. All Spectro. 79, 888 (2013).
Kroupa, J., Schranz, W., Fuith, A. & Warhanek, H. Saint-Gregoire, P. Study of the linear birefringence and domain structure in [N(CH3)4]2CoCl4 and [N(CD3)4]2ZnCl4. Ferroelectrics 125, 165 (1992).
Folcia, C. L. & Perez-Mato, J. M. Nonsoliton model for dielectric anomalies at the incommensurate-ferroelectric phase transitions in [N(CH3)4]2XCl4 (X = Co, Zn). Phys Rev B. 42, 8499 (1990).
Folcia, C. L., Perez-Mato, J. M., Zuniga, F. J., Madariaga, G. & Tello, M. J. A model for the dielectric behaviour of [N(CH3)4]2XCl4 with X = Co, Zn. Ferroelectrics 105, 303 (1990).
Lim, A. R. Roles of chemically inequivalent N(CH3)4 ions in phase transition temperatures in [N(CH3)4]2CoCl4 by single-crystal NMR and MAS NMR. Chem. Phys. 436–437, 46 (2014).
Lim, A. R. Nuclear magnetic resonance in [N(CH3)4]2CoCl4 single crystals: transferred hyperfine interaction and spin-lattice relaxation rate. J. Phys. Chem. Solids. 66, 973 (2005).
Lim, A. R. Changing structural properties of mixed crystals [N(CH3)4]2Zn1-xCoxCl4 (x = 0, 0.5, 0.7, 0.9, and 1) by magic angle spinning nuclear magnetic resonance. Mater. Chem. Phys. 171, 379 (2016).
Lim, A. R. Ferroelastic phase transitions by 14N NMR spectra in [N(CH3)4]2CoCl4 and [N(CH3)4]2ZnCl4 single crystals. Solid State Commun. 242, 25 (2016).
Lim, A. R. & Jung, W. K. Proton magnetic resonance study of the phase transitions of [N(CH3)4]2BCl4 (B = 59Co, 63Cu, 67Zn, and 113Cd) single crystals. J. Phys. Chem. Solids. 66, 1795 (2005).
Poprawski, R., Liber, A. & Malek, E. Dilatometric investigations of overcritical behavious in [N(C2H5)4]2CuCl4 crystals. Acta Phys. Polonica A. 98, 61 (2000).
Tylczynski, Z., Biskupski, P. & Slaboszewska, M. Dielectric dispersion in [N(C2H5)4]2MeCl4 crystals. Ferroelectrics 272, 315 (2002).
Kandhaswamy, M. A. & Srinivasan, V. Synthesis and characterization of Tetrarthylammonium Tetrachlorocobaltate crystals. Bull. Mater. Sci. 25, 41 (2002).
Gesi, K. Effect of hydrostatic pressure on the phase transitions in [N(C2H5)4]2CuCl4. Ferroelectrics 285, 139 (2003).
Biskupski, P., Slaboszewska, M. & Tylczynski, Z. Changes in the optical properties at phase transitions in TEA2MeCl4 (Me = Zn, Mn, Hg, Cu) crystals. Phys B. 370, 6 (2005).
Sheleg, A. U., Natumovets, A. M., Dekola, T. I. & Tekhanovich, N. P. Effect of γ irradiation on the structural and thermal properties of [N(C2H5)4]2ZnBr4 in the vicinity of a first-order phase transition. Phys. Solid State. 48, 354 (2006).
Maczka, M., Cizman, A., Poprawski, R. & Hanuza, J. Temperature-dependent vibrational studies of [N(C2H5)4]2MnCl4. J. Raman Spectrosc. 38, 1622 (2007).
Sheleg, A. U., Zub, E. M. & Yachkovskii, A. Y. Crystallographic characteristics and phase transitions in the [N(C2H5)4]2CdBr4 crystal in the low-temperature range. Phys. Solid State. 49, 1973 (2007).
Dekola, T. I., Sheleg, A. U. & Tekhanovich, N. P. Heat capacity of the [N(C2H5)4]2CdBr4 crystal in the temperature range 80–300 K. Phys. Solid State. 49, 1766 (2007).
Lim, A. R. & Lim, K. Y. Phase-transition mechanisms of [N(C2H5)4]2BCl4 and [N(CH3)4]2BCl4 (B = 63Cu and 67Zn) single crystals studied by proton NMR. Solid State Commun. 147, 11 (2008).
Biskupski, P. & Tylczynski, Z. Structure of TEA2ZnCl4 crystal surfaces studied by AFM. Phase Transition. 81, 971 (2008).
Ostrowski, A. & Cizman, A. EPR studies of linewidth anomalies at phase transitions in [N(C2H5)4]2MnCl4. Physica B. 403, 3110 (2008).
Lim, A. R. Study on Ethyl groups with two different orientations in [N(C2H5)4]2CuBr4. J. Phys. Chem. Solids. 93, 59 (2016).
Lim, A. R. Study of the ferroelastic phase transition in the Tetraethylammonium compound [N(C2H5)4]2ZnBr4 by magic-angle spinning and static NMR. AIP Adv. 6, 035307 (2016).
Lim, A. R., Kim, M. S. & Lim, K. Y. Nuclear magnetic resonance study of the ferroelastic phase transition of order-disorder type [N(C2H5)4]2CdCl4. Solid State Sci. 58, 101 (2016).
Lim, A. R. & Lim, K. Y. Structural changes near phase transition temperatures for the [N(C2H5)4] groups in hydrated [N(C2H5)4]2CuCl4∙xH2O. J. Therm. Anal. Calorim. 130, 879 (2017).
Bechir, M. B., Rhaiem, A. M. & Synthesis Thermal analysis, optical, electric properties and conduction mechanism of hybrid halogenometallates: [N(C2H5)4]2CoCl4. J. Phys. Soc. Japan. 90, 74709 (2021).
Souissi, H. et al. Experimental and optical studies of the new organic inorganic bromide: [(C3H7)4N]2CoBr4. Opt. Mater. 129, 112513 (2022).
Gzaiel, M. B., Oueslati, A., Hlel, F. & Gargouri, M. Synthesis, crystal structure, phase transition and electrical conduction mechanism of the new [(C3H7)4N]2MnCl4 compound. Physica E. 83, 405 (2016).
Banupriya, K., Revathi, A., Sudha, D., Kirubavathy, S. J. & Umarani, R. Tetra-propylammonium tribromocuprate complex[(C3H7)4N]CuBr3(II)-synthesis, thermal and spectral characterization. Mater. Today: proceedings. 45, 8024 (2021).
Moutia, N., Oueslati, A., Gzaiel, M. B. & Khirouni, K. Crystal structure and AC conductivity mechanism of [N(C3H7)4]2CoCl4 compound. Physica E. 83, 88 (2016).
Chkoundali, S., Hlel, F. & Khemekhem, H. Synthesis, crystal structure, thermal and dielectric properties of tetrapropylammonium tetrabromozincate [N(C3H7)4]2[ZnBr4] compound. Appl Phys A. 122, 1066 (2016).
Moutia, N., Gzaiel, M. B., Oueslati, A. & Khirouni, K. Electric characterization and vibrational spectroscopic investigations of order-disorder phase transitions in [N(C3H7)4]2CoCl4 compound. J. Mol. Struct. 1134, 697 (2017).
Chkoundali, S. & Aydi, A. Electrical conductivity and vibrational studies induced phase transition in [N(C3H7)4]2ZnBr4. J. Adv. Dielectr. 11, 2150005 (2021).
Khalfa, M. et al. Synthesis, structural and electrical characterization of a new organic inorganic bromide: [(C3H7)4N]2CoBr4. RSC Adv. 12, 2798 (2022).
Taktak, O. et al. Optical investigations and theoretical simulation of organic-inorganic hybrid: TPA-CoCl4. Opt. Mater. 150, 115251 (2024).
Khalfa, M. et al. New organic-inorganic bromides [N(C3H7)4]2MBr4 (M = Hg and Cd): synthesis, crystal structure and vibrational characterization. J. Alloy Compd. 6, 181334 (2025).
Kanagarajan, B., Parveen, S., Ramasamy, R. & Ramasamy, U. New tetrapropyl-ammonium tetrabromozincate complex [N(C3H7)4]2ZnBr4 (II)-synthesis, spectral, thermal characterization and antioxidant activity. Bull. Chem. Soc. Ethiop. 37, 623 (2023).
Oueslati, A. et al. Infrared, polarized Raman and Ab initio calculations of the vibrational spectra of [N(C3H7)4]2Cu2Cl6 crystals. Vib. Spectrosc. 64, 10 (2013).
Dhouib, I., Guionneau, P., Pechev, S., Mhiri, T. & Elaoud, Z. Crystal structure and spectroscopic study of bis-tetrapropylammonium hexachlorodicuprate (II), [N(C3H7)4]2Cu2Cl6. Eur. J. Chem. 4, 117 (2013).
Mbarek, I. et al. Unraveling the properties of [TPA]2Cu2Br6: A holistic investigation into structure, optics, magnetism and dielectric characteristics. J. Mol. Struct. 1321, 140115 (2025).
Oueslati, A. & Gargouri, M. Studies on structural, electrical, and transport properties of [(C3H7)4N]2Cu2Br6 compound. J. Alloys Compd. 739, 1089 (2018).
Hannachi, N., Bulou, A., Chassenieux, C., Guidara, K. & Hlel, F. Temperature study of [N(C3H7)4]2Cd2Cl6 by thermal analysis, Raman scattering, and X-ray powder diffraction: evidence of phase transitions. Physica A. 390, 2987 (2011).
Hannachi, N., Guidara, K., Bulou, A., Gargouri, M. & Hlel, F. Polarized Raman study of [N(C3H7)4]2Cd2Cl6 single crystal. Spectrochimica A. 77, 457 (2010).
Hannachi, N., Chaabane, I., Guidara, K., Bulou, A. & Hlel, F. AC electrical properties and dielectric relaxation of [N(C3H7)4]2Cd2Cl6 single crystal. Mater Sci. Engineering. B 172, 24 (2010).
Hannachi, N., Guidara, K., Bulou, A. & Hlel, F. Structural characterization and AC conductivity of Bis tetrapropylammonium hexachlorado-dicadmate, [N(C3H7)4]2Cd2 Cl6. Mater. Res. Bull. 45, 1754 (2010).
Dhouib, I., Guionneau, P., Pechev, S., Mhiri, T. & Elaoud, Z. Crystal structure and spectroscopic study of bis-tetrapropylammonium hexachlorodicuprate (II), [N(C3H7)4]2Cu2Cl6. Euro. J. Chem. 4, 117 (2013).
Oueslati, A. et al. Infrared, polarized Raman and Ab initio calculations of the vibrational spectra of [N(C3H7)4]2Cu2Cl6 crystals. Vib. Spectro. 64, 10 (2013).
Gzaiel, M. B. et al. Synthesis, crystal structure, thermal analysis, and electrical properties of Bis tetrapropylammonium hexachloro-dizincate compound. Ionics 20, 221 (2014).
Gzaiel, M. B., Oueslati, A. & Gargour, M. Ac conductivity and transport properties of [N(C3H7)4]2Zn2Cl6 compound. J. Clust Sci. 26, 1577 (2015).
Gzaiel, M. B. et al. Using Raman spectroscopy to understand the origin of the phase transitions observed in [(C3H7)4N]2Zn2Cl6 compound. Spectro Acta A 145, 223 (2015).
SMART and SAINT-Plus v6.22. Bruker AXS Inc., Madison, Wisconsin, USA, (2000).
Koenig, J. L. Spectroscopy of Polymers (Elsevier, 1999).
Abragam, A. The Principles of Nuclear Magnetism (Oxford University Press, 1961).
Acknowledgements
This research was supported by the Regional Innovation System & Education (RISE) program through the Jeonbuk RISE Center, funded by the Ministry of Education (MOE) and the Jeonbuk State, Republic of Korea (2025-RISE-13-JJU).
Funding
This research was supported by the Regional Innovation System & Education (RISE) program through the Jeonbuk RISE Center, funded by the Ministry of Education (MOE) and the Jeonbuk State, Republic of Korea (2025-RISE-13-JJU).
Author information
Authors and Affiliations
Contributions
A.R. Lim designed the project, NMR experiment, and wrote the manuscript. H. Ju performed the SCXRD measurements, Y. S. Shin carried out the DSC, TGA, and optical microscopy experiment.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ju, H., Shin, Y.S. & Lim, A.R. A comprehensive study of the crystal structure and dynamics of [N(C3H7)4]2Cd2Cl6. Sci Rep (2026). https://doi.org/10.1038/s41598-026-35886-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-35886-8