Abstract
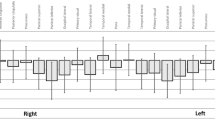
Despite established links between masticatory dysfunction and cognitive impairment, cognitive function in dentofacial deformity patients remains unexplored. This study represents the first comprehensive cognitive assessment in mandibular prognathism (MP) patients. Brain blood flow (BBF) during chewing was measured using functional near-infrared spectroscopy in MP patients versus normal occlusion controls (NORM, n = 17). Cognitive function using eye-tracking technology (Mirudake) in MP patients (n = 44) was compared to healthy controls (HC, n = 59), with assessing six cognitive domains. MP patients demonstrated significantly reduced chewing-induced BBF in bilateral inferior frontal gyrus compared to NORM. Global cognitive performance showed no significant difference between MP and HC groups (p = 0.48). However, positive correlations existed between BBF and cognitive performance domains including global performance and memory. ROC analysis using pooled bilateral BBF data (n = 88) revealed modest diagnostic potential for cognitive assessment (AUC = 0.657, 95% CI 0.431–0.865). The optimal threshold yielded 62.5% sensitivity, 73.8% specificity, and 72.7% overall accuracy for detecting cognitive impairment. This first systematic cognitive evaluation of MP patients revealed no significant global cognitive impairment despite confirmed reductions in masticatory-induced brain activation. The observed BBF-cognition correlations suggest BBF measurements may have potential utility as an adjunct measure in cognitive screening, warranting further investigation through longitudinal studies examining orthognathic surgery’s potential cognitive effects.
Similar content being viewed by others
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
References
Proffit, W. R., Fields, H. W. Jr. & Moray, L. J. Prevalence of malocclusion and orthodontic treatment need in the United States: estimates from the NHANES III survey. Int. J. Adult Orthodon. Orthognath. Surg. 13, 97–106 (1998).
van den Braber, W., van der Glas, H., van der Bilt, A. & Bosman, F. Masticatory function in retrognathic patients, before and after mandibular advancement surgery. J Oral Maxillof Surg: official J Amer Assoc Maxillo Surg 62, 549–554. https://doi.org/10.1016/j.joms.2003.06.016 (2004).
Iwase, M., Ohashi, M., Tachibana, H., Toyoshima, T. & Nagumo, M. Bite force, occlusal contact area and masticatory efficiency before and after orthognathic surgical correction of mandibular prognathism. Int. J. Oral Maxillofac. Surg. 35, 1102–1107. https://doi.org/10.1016/j.ijom.2006.08.014 (2006).
Kobayashi, T., Honma, K., Shingaki, S. & Nakajima, T. Changes in masticatory function after orthognathic treatment in patients with mandibular prognathism. Br. J. Oral Maxillofac. Surg. 39, 260–265. https://doi.org/10.1054/bjom.2000.0576 (2001).
Nakata, Y. et al. Changes in stomatognathic function induced by orthognathic surgery in patients with mandibular prognathism. J Oral Maxillof Surg : Off J Am Assoc Oral Maxillof Surg 65, 444–451. https://doi.org/10.1016/j.joms.2005.12.071 (2007).
Weijenberg, R. A., Lobbezoo, F., Knol, D. L., Tomassen, J. & Scherder, E. J. Increased masticatory activity and quality of life in elderly persons with dementia: a longitudinal matched cluster randomized single-blind multicenter intervention study. BMC Neurol 13, 26. https://doi.org/10.1186/1471-2377-13-26 (2013).
Kossioni, A. E. The association of poor oral health parameters with malnutrition in older adults: A review considering the potential implications for cognitive impairment. Nutrients https://doi.org/10.3390/nu10111709 (2018).
Lexomboon, D., Trulsson, M., Wårdh, I. & Parker, M. G. Chewing ability and tooth loss: Association with cognitive impairment in an elderly population study. J Am Geriatr Soc 60, 1951–1956. https://doi.org/10.1111/j.1532-5415.2012.04154.x (2012).
Yamamoto, T. et al. Association between self-reported dental health status and onset of dementia: A 4-year prospective cohort study of older Japanese adults from the Aichi Gerontological evaluation study (AGES) Project. Psychosom. Med. 74, 241–248. https://doi.org/10.1097/PSY.0b013e318246dffb (2012).
Luraschi, J. et al. Neuroplasticity in the adaptation to prosthodontic treatment. J Orofac Pain 27, 206–216. https://doi.org/10.11607/jop.1097 (2013).
Sesay, M., Tanaka, A., Ueno, Y., Lecaroz, P. & De Beaufort, D. G. Assessment of regional cerebral blood flow by xenon-enhanced computed tomography during mastication in humans. Keio J Med 49(Suppl 1), A125-128 (2000).
Momose, T. et al. Effect of mastication on regional cerebral blood flow in humans examined by positron-emission tomography with 15O-labelled water and magnetic resonance imaging. Arch. Oral Biol. 42, 57–61. https://doi.org/10.1016/s0003-9969(96)00081-7 (1997).
Kanzaki, H. et al. Mandibular prognathism attenuates brain blood flow induced by chewing. Sci Rep 9, 19104. https://doi.org/10.1038/s41598-019-55553-5 (2019).
Kariya, C. et al. Skeletal anterior open bite attenuates the chewing-related increase in brain blood flow. Dent J 12, 161 (2024).
Maillet, D. & Rajah, M. N. Association between prefrontal activity and volume change in prefrontal and medial temporal lobes in aging and dementia: A review. Ageing Res. Rev. 12, 479–489. https://doi.org/10.1016/j.arr.2012.11.001 (2013).
Nyberg, L. Cognitive control in the prefrontal cortex: A central or distributed executive?. Scand. J. Psychol. 59, 62–65. https://doi.org/10.1111/sjop.12409 (2018).
Galindo-Moreno, P. et al. The impact of tooth loss on cognitive function. Clin. Oral Invest. 26, 3493–3500. https://doi.org/10.1007/s00784-021-04318-4 (2022).
Saito, S. et al. Association between tooth loss and cognitive impairment in community-dwelling older Japanese adults: A 4-year prospective cohort study from the Ohasama study. BMC Oral Health 18, 142. https://doi.org/10.1186/s12903-018-0602-7 (2018).
Okamoto, N. et al. Association between tooth loss and the development of mild memory impairment in the elderly: The Fujiwara-kyo Study. J Alzheimers Dis 44, 777–786. https://doi.org/10.3233/jad-141665 (2015).
Kiuchi, S. et al. Oral status and dementia onset: mediation of nutritional and social factors. J. Dent. Res. 101, 420–427. https://doi.org/10.1177/00220345211049399 (2022).
Pachêco-Pereira, C., Pereira, J. R., Dick, B. D., Perez, A. & Flores-Mir, C. Factors associated with patient and parent satisfaction after orthodontic treatment: a systematic review. Am J Orthod Dentofac Orthop: Off Publ Am Assoc Orthod, Const Soc, Amer Orthod 148, 652–659. https://doi.org/10.1016/j.ajodo.2015.04.039 (2015).
Soh, C. L. & Narayanan, V. Quality of life assessment in patients with dentofacial deformity undergoing orthognathic surgery: A systematic review. Int. J. Oral Maxillofac. Surg. 42, 974–980. https://doi.org/10.1016/j.ijom.2013.03.023 (2013).
Abrahamsson, C., Henrikson, T., Bondemark, L. & Ekberg, E. Masticatory function in patients with dentofacial deformities before and after orthognathic treatment-a prospective, longitudinal, and controlled study. Eur. J. Orthod. 37, 67–72. https://doi.org/10.1093/ejo/cju011 (2015).
Oyama, A. et al. Novel method for rapid assessment of cognitive impairment using high-performance eye-tracking technology. Sci Rep 9, 12932. https://doi.org/10.1038/s41598-019-49275-x (2019).
Wolf, A., Tripanpitak, K., Umeda, S. & Otake-Matsuura, M. Eye-tracking paradigms for the assessment of mild cognitive impairment: a systematic review. Front Psychol 14, 1197567. https://doi.org/10.3389/fpsyg.2023.1197567 (2023).
Stuart, S. et al. The measurement of eye movements in mild traumatic brain injury: A structured review of an emerging area. Front Sports Act Living 2, 5. https://doi.org/10.3389/fspor.2020.00005 (2020).
Opwonya, J. et al. Saccadic eye movement in mild cognitive impairment and Alzheimer’s disease: A systematic review and meta-analysis. Neuropsychol Rev 32, 193–227. https://doi.org/10.1007/s11065-021-09495-3 (2022).
Semmelmann, K. & Weigelt, S. Online webcam-based eye tracking in cognitive science: A first look. Behav Res Methods 50, 451–465. https://doi.org/10.3758/s13428-017-0913-7 (2018).
Cuve, H. C., Stojanov, J., Roberts-Gaal, X., Catmur, C. & Bird, G. Validation of Gazepoint low-cost eye-tracking and psychophysiology bundle. Behav Res Methods 54, 1027–1049. https://doi.org/10.3758/s13428-021-01654-x (2022).
Kaufmann, B. C. et al. Eyetracking during free visual exploration detects neglect more reliably than paper-pencil tests. Cortex 129, 223–235. https://doi.org/10.1016/j.cortex.2020.04.021 (2020).
Dintica, C. S. et al. The relation of poor mastication with cognition and dementia risk: a population-based longitudinal study. Aging 12, 8536–8548. https://doi.org/10.18632/aging.103156 (2020).
Sta Maria, M. T., Hasegawa, Y., Khaing, A. M. M., Salazar, S. & Ono, T. The relationships between mastication and cognitive function: A systematic review and meta-analysis. Jpn Dent Sci Rev 59, 375–388. https://doi.org/10.1016/j.jdsr.2023.10.001 (2023).
Mouelhi, Y., Jouve, E., Castelli, C. & Gentile, S. How is the minimal clinically important difference established in health-related quality of life instruments? Review of anchors and methods. Health Qual. Life Outcomes 18, 136. https://doi.org/10.1186/s12955-020-01344-w (2020).
Instructions for Use for Controlled Medical Device, <https://www.pmda.go.jp/PmdaSearch/kikiDetail/ResultDataSetPDF/113053_30500BZX00235000_A_01_04> (
Saito, T. & Rehmsmeier, M. The precision-recall plot is more informative than the ROC plot when evaluating binary classifiers on imbalanced datasets. PLoS ONE 10, e0118432. https://doi.org/10.1371/journal.pone.0118432 (2015).
Mao, K. et al. Accuracy of near-infrared reflection in detecting proximal caries: A systematic review and meta-analysis. J Dent 161, 105949. https://doi.org/10.1016/j.jdent.2025.105949 (2025).
Sasaki, Y. et al. Association between severity of malocclusion and parameters of oral functions in permanent dentition with various malocclusion: Case-control study. Clin Investig Orthod https://doi.org/10.1080/27705781.2023.2267847 (2023).
Kim, S. et al. Effect of chewing hard material on boosting brain antioxidant levels and enhancing cognitive function. Front Syst Neurosci 18, 1489919. https://doi.org/10.3389/fnsys.2024.1489919 (2024).
Kulchutisin, P., Sowithayasakul, T., Pumklin, J. & Piyapattamin, T. Electromyographic evaluations of masticatory muscle activity between patients with skeletal class I and III relationships. Eur J Dent 17, 910–916. https://doi.org/10.1055/s-0042-1758064 (2023).
Sessle, B. J. Mechanisms of oral somatosensory and motor functions and their clinical correlates. J. Oral Rehabil. 33, 243–261. https://doi.org/10.1111/j.1365-2842.2006.01623.x (2006).
Avivi-Arber, L. & Sessle, B. J. Jaw sensorimotor control in healthy adults and effects of ageing. J. Oral Rehabil. 45, 50–80. https://doi.org/10.1111/joor.12554 (2018).
Avivi-Arber, L. et al. Widespread volumetric brain changes following tooth loss in female mice. Front Neuroanat 10, 121. https://doi.org/10.3389/fnana.2016.00121 (2016).
Lin, C. S., Wu, S. Y., Wu, C. Y. & Ko, H. W. Gray matter volume and resting-state functional connectivity of the motor cortex-cerebellum network reflect the individual variation in masticatory performance in healthy elderly people. Front Aging Neurosci 7, 247. https://doi.org/10.3389/fnagi.2015.00247 (2015).
Kubo, K. Y. et al. Tooth loss early in life suppresses neurogenesis and synaptophysin expression in the hippocampus and impairs learning in mice. Arch. Oral Biol. 74, 21–27. https://doi.org/10.1016/j.archoralbio.2016.11.005 (2017).
Kesner, R. P., Farnsworth, G. & Kametani, H. Role of parietal cortex and hippocampus in representing spatial information. Cereb Cortex 1, 367–373. https://doi.org/10.1093/cercor/1.5.367 (1991).
Nitz, D. A. Tracking route progression in the posterior parietal cortex. Neuron 49, 747–756. https://doi.org/10.1016/j.neuron.2006.01.037 (2006).
Baker, A. E., Galván, A. & Fuligni, A. J. The connecting brain in context: How adolescent plasticity supports learning and development. Dev Cogn Neurosci 71, 101486. https://doi.org/10.1016/j.dcn.2024.101486 (2025).
Larsen, B. & Luna, B. Adolescence as a neurobiological critical period for the development of higher-order cognition. Neurosci Biobehav Rev 94, 179–195. https://doi.org/10.1016/j.neubiorev.2018.09.005 (2018).
Poletti, B. et al. An eye-tracking controlled neuropsychological battery for cognitive assessment in neurological diseases. Neurol Sci 38, 595–603. https://doi.org/10.1007/s10072-016-2807-3 (2017).
Athanasiou, A. E., Melsen, B., Mavreas, D. & Kimmel, F. P. Stomatognathic function of patients who seek orthognathic surgery to correct dentofacial deformities. Int. J. Adult Orthodon. Orthognath. Surg. 4, 239–254 (1989).
Ingervall, B. & Helkimo, E. Masticatory muscle force and facial morphology in man. Arch. Oral Biol. 23, 203–206. https://doi.org/10.1016/0003-9969(78)90217-0 (1978).
Chujo, M., Sugawara, J., Tomoyose, Y., Kawamura, H. & Mitani, H. effects of functional training with chewing gum after surgical orthodontic treatment on masticatory system in jaw deformity patients. Jpn J Jaw Deform 14, 170–179. https://doi.org/10.5927/jjjd1991.14.170 (2004).
Acknowledgements
The authors thank Ai-BrainScience Inc. (Osaka, Japan) for generously providing cognitive function data from healthy control participants. This work was supported in part by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (23K09449 and 24K13186).
Funding
This work was supported in part by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (23K09449 and 24K13186).
Author information
Authors and Affiliations
Contributions
Y.I.: Acquisition and analysis of data. Revised the manuscript. H.K.: Conception and design of this work. Interpretation of data. Have drafted the manuscript. C.K.: Design of this work. Acquisition and analysis of data. Revised the manuscript. S.T.: Acquisition of data. Revised the manuscript. M.K.: Conception and design of this work. Revised the manuscript. H.T.: Revised the manuscript.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Inagawa, Y., Kanzaki, H., Kariya, C. et al. Association between reduced chewing-induced brain blood flow and cognitive performance in mandibular prognathism patients in a pilot study. Sci Rep (2026). https://doi.org/10.1038/s41598-026-35964-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-35964-x