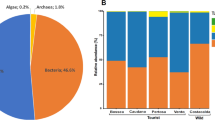
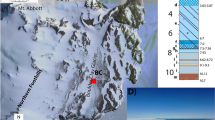
Abstract
Anchialine caves house a vast variety of organisms that support complex ecological relationships among themselves and their environment. The following study was made in the anchialine karst cave El Aerolito, found on Cozumel Island, Quintana Roo, Mexico. It explores the relationship between wall microbial mats and the diet of Asterinides sp., an endemic stygobitic seastar. Wall microbial mats inside the cave were sampled and the stomach microbiome of Asterinides sp. was obtained through regurgitation. Asterinides sp. sampling was made through the Catcher Collection Chamber (CCC), an innovative technology for the exploration of these ecosystems. The obtained results suggest that microbial mats are part of the diet of Asterinides sp. The following results highlight the potential relevance of the microbial communities inside the trophic chain present in El Aerolito. Additionally, the methodology presented here provides a useful framework for future ecological research in El Aerolito cave.
Similar content being viewed by others
Data availability
The sequence datasets that support this study are available on NCBI Sequence Read Archive (SRA), under BioProject number PRJNA1119047 (https://www.ncbi.nlm.nih.gov/sra/PRJNA1119047) ; individual accession numbers: SAM41635988, SAM41635989, SAM41635990, SAM41635991, SAM41635992, SAM41635993, SAM41635994, SAM41635995, SAM41635996, SAM41635997, SAM41635998, SAM41635999. The data generated during the current study are included in the supplementary material.
References
Estrada Medina, H., Jiménez Osornio, J. J. & Álvarez-Rivera, O. Barrientos Medina, R. C. El karst de Yucatán: Su origen, morfología y biología. Acta Univ. 29, 1–18. https://doi.org/10.15174/au.2019.2292 (2019).
Calderón-Gutiérrez, F., Sánchez-Ortiz, C. A. & Huato-Soberanis, L. Ecological patterns in anchialine caves. PLoS One. 13, e0202909. https://doi.org/10.1371/journal.pone.0202909 (2018).
Solís-Marín, F. A., Laguarda-Figueras, A., Gutierrez, F. V., Mejía, L. & Yáñez, G. Echinoderm fauna of anchialine caves in Cozumel Island, Mexico. Echinoderms: Durham-Proceedings of the 12th International Echinoderm Conference, 259–261. https://doi.org/10.1201/9780203869543-c42 (2010).
Márquez-Borrás, F. et al. & Mejía-Ortiz, L. M. Troglomorphism in the brittle star Ophionereis commutabilis Bribiesca-Contreras et al., 2019 (Echinodermata, Ophiuroidea, Ophionereididae), Subterr Biol. 33, 87–108. https://doi.org/10.3897/subtbiol.33.48721 (2020).
Reddell, J. R. A review of the cavernicole fauna of Mexico, Guatemala, and Belize. Bull. Tex. Mem. Mus. Univ. Tex. Austin. 27, 1–327 (1981).
Bribiesca-Contreras, G., Solís-Marín, F. A., Laguarda-Figueras, A. & Zaldívar-Riverón, A. Identification of echinoderms (Echinodermata) from an anchialine cave in Cozumel Island, Mexico, using DNA barcodes. Mol. Ecol. Resour. 13, 1137–1145. https://doi.org/10.1111/1755-0998.12098 (2013).
Jangoux, M. Food and feeding mechanisms: Asteroidea. In Echinorderm Nutrition, vol. 1 (eds Jangoux, M. & Lawrence, J. M.) 117–159 (CRC, 1982).
Brankovits, D. et al. Changes in organic matter deposition can impact benthic marine meiofauna in karst subterranean estuaries. Front. Environ. Sci. 9 https://doi.org/10.3389/fenvs.2021.670914 (2021).
Zhu, H. Z. et al. Bacteria and metabolic potential in karst caves revealed by intensive bacterial cultivation and genome assembly. Appl. Environ. Microbiol. 87, e02440–e02420. https://doi.org/10.1128/AEM.02440-20 (2021).
Kajan, K., Cukrov, N., Cukrov, N., Bishop-Pierce, R. & Orlić, S. Microeukaryotic and prokaryotic diversity of anchialine caves from Eastern Adriatic sea Islands. Microb. Ecol. 83, 257–270. https://doi.org/10.1007/s00248-021-01760-5 (2022).
Tetu, S. G. et al. Life in the dark: metagenomic evidence that a microbial slime community is driven by inorganic nitrogen metabolism. ISME J. 7, 1227–1236. https://doi.org/10.1038/ismej.2013.14 (2013).
Por, F. D. Ophel: a groundwater biome based on chemoautotrophic resources. The global significance of the Ayyalon cave finds. Isr. Hydrobiol. 592, 1–10. https://doi.org/10.1007/s10750-007-0795-2 (2007).
Montano, E. T. & Henderson, L. O. Studies of antibiotic production by cave Bacteria. In Cave Microbiomes: A Novel Resource for Drug Discovery. Springer Brief in Microbiology (ed. Cheeptham N.) 109–130. https://doi.org/10.1007/978-1-4614-5206-5_6 (2013).
Archana, A., Francis, C. A. & Boehm, A. B. The beach aquifer microbiome: research gaps and data needs. Front. Environ. Sci. 9 https://doi.org/10.3389/fenvs.2021.653568 (2021).
Simon, K. S., Benfield, E. F. & Macko, S. A. Food web structure and the role of epilithic biofilms in cave streams. Ecology. 84, 2395–2406 (2003). https://doi.org/10.1890/02-334 2206–2232 (2021). https://doi.org/10.1038/s41396-021-00917-x.
Brankovits, D. & Pohlman, J. W. Methane oxidation dynamics in a karst subterranean estuary. Geochim. Cosmochim. Acta. 277, 320–333. https://doi.org/10.1016/j.gca.2020.03.007 (2020).
Menning, D. M. et al. Aquifer discharge drives microbial community change in karst estuaries. Estuaries Coast. 41, 430–443. https://doi.org/10.1007/s12237-017-0281-7 (2018).
Patin, N. V. et al. Gulf of Mexico blue hole harbors high levels of novel microbial lineages. ISME J. 15, 2206–2232. https://doi.org/10.1038/s41396-021-00917-x (2021).
Medina-Tanco, G. et al. A new collection method for Asterinides sp., a troglobitic seastar inhabiting the anchialine karst system El Aerolito, Cozumel Island, Mexico. (2021). Unpublished data.
Comeau, A. M., Li, W. K. W., Tremblay, J. É., Carmack, E. C. & Lovejoy, C. Arctic ocean microbial community structure before and after the 2007 record sea ice minimum. PLoS One. 6, e27492. https://doi.org/10.1371/journal.pone.0027492 (2011).
Callahan, B. J. et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods. 13, 581–583. https://doi.org/10.1038/nmeth.3869 (2016).
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596. https://doi.org/10.1093/nar/gks1219 (2012).
Oksanen, J. et al. Vegan: Community ecology package. R package version 2.6-4. CRAN (2022). https://CRAN.R-project.org/package=vegan
McMurdie, P. J. & Holmes, S. Phyloseq: An R package for reproducible interactive analysis and graphics of Microbiome census data. PLoS One. 8, e61217. https://doi.org/10.1371/journal.pone.0061217 (2013).
Lahti, L. et al. Tools for microbiome analysis in R. Microbiome package version 1.31.2. Bioconductor, 2017. https://github.com/microbiome/microbiome
Wickham, H. et al. Welcome to the tidyverse. J. Open. Source Softw. 4, 1686. https://doi.org/10.21105/joss.01686 (2019).
Lozupone, C. & Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 71, 8228–8235. https://doi.org/10.1128/AEM.71.12.8228-8235.2005 (2005).
Wemheuer, F. et al. Tax4Fun2: prediction of habitat-specific functional profiles and functional redundancy based on 16S rRNA gene sequences. Environ. Microbiome. 15, 11. https://doi.org/10.1186/s40793-020-00358-7 (2020).
Jurado, V. et al. Dominance of Arcobacter in the white filaments from the thermal sulfidic spring of fetida cave (Apulia, Southern Italy). Sci. Total Environ. 800, 149465. https://doi.org/10.1016/j.scitotenv.2021.149465 (2021).
Brankovits, D. et al. Methane-and dissolved organic carbon-fueled microbial loop supports a tropical subterranean estuary ecosystem. Nat. Commun. 8, 1835. https://doi.org/10.1038/s41467-017-01776-x (2017).
Calderón-Gutiérrez, F., Iliffe, T. M., Borda, E., Yáñez Mendoza, G. & Labonté, J. Response and resilience of karst subterranean estuary communities to precipitation impacts. Ecol. Evol. 13, e10415. https://doi.org/10.1002/ece3.10415 (2023).
Lee, H. R., Park, S. H., Kim, D. H., Moon, K. M. & Heo, M. S. Microbial community analysis isolated from red starfish (Certonardoa semiregularis) gut. J. Life Sci. 28, 955–961 (2018).
Choi, G. G., Lee, O. H. & Lee, G. H. The diversity of heterotrophic bacteria isolated from intestine of starfish (Asterias amurensis) by analysis of 16S rDNA sequence. Korean J. Ecol. 26, 307–312 (2003).
Okazaki, Y. et al. Microdiversity and phylogeographic diversification of bacterioplankton in pelagic freshwater systems revealed through long-read amplicon sequencing. Microbiome 9, 24. https://doi.org/10.1186/s40168-020-00974-y (2021).
Zhou, L. et al. Environmental filtering dominates bacterioplankton community assembly in a highly urbanized estuarine ecosystem. Environ. Res. 196, 110934. https://doi.org/10.1016/j.envres.2021.110934 (2021).
Ghylin, T. W. et al. Comparative single-cell genomics reveals potential ecological niches for the freshwater aci actinobacteria lineage. ISME J. 8, 2503–2516. https://doi.org/10.1038/ismej.2014.135 (2014).
Zufiaurre, A. et al. Bacterioplankton seasonality in deep high-mountain lakes. Front. Microbiol. 13, 935378. https://doi.org/10.3389/fmicb.2022.935378 (2022).
He, H. et al. Community structure, abundance and potential functions of bacteria and archaea in the Sansha Yongle blue Hole, Xisha, South China sea. Front. Microbiol. 10, 2404. https://doi.org/10.3389/fmicb.2019.02404 (2019).
Stoessell, R. K., Moore, Y. H. & Coke, J. G. The occurrence and effect of sulfate reduction and sulfide oxidation on coastal limestone dissolution in Yucatan cenotes. Groundwater 31, 566–575. https://doi.org/10.1111/j.1745-6584.1993.tb00589.x (1993).
Parrilli, E. et al. The Art of adapting to extreme environments: the model system Pseudoalteromonas. Phys. Life Rev. 36, 137–161. https://doi.org/10.1016/j.plrev.2019.04.003 (2021).
Jensen, S. et al. The relative abundance and transcriptional activity of marine sponge-associated microorganisms emphasizing groups involved in sulfur cycle. Microb. Ecol. 73, 668–676. https://doi.org/10.1007/s00248-016-0836-3 (2017).
Zhou, Q., Jia, L., Wu, W. & Wu, W. Introducing PHBV and controlling the pyrite sizes achieved the pyrite-based mixotrophic denitrification under natural aerobic conditions: low sulfate production and functional microbe interaction. J. Clean. Prod. 366, 132986. https://doi.org/10.1016/j.jclepro.2022.132986 (2022).
Moore, A. Characterization of the native microbial communities in the karst aquifer of Yucatan Peninsula, Mexico. Graduate Research Theses & Dissertations. 1753 (2014).
Durham, B. P. et al. Draft genome sequence of marine alphaproteobacterial strain HIMB11, the first cultivated representative of a unique lineage within the Roseobacter clade possessing an unusually small genome. Stand. Genomic Sci. 9, 632–645. https://doi.org/10.4056/sigs.4998989 (2014).
Bauermeister, J., Ramette, A. & Dattagupta, S. Repeatedly evolved host-specific ectosymbioses between sulfur-oxidizing bacteria and amphipods living in a cave ecosystem. PLoS One. 7, e50254. https://doi.org/10.1371/journal.pone.0050254 (2012).
Brigmon, R. L. & De Ridder, C. Symbiotic relationship of Thiothrix spp. with an echinoderm. Appl. Environ. Microbiol. 64, 3491–3495. https://doi.org/10.1128/AEM.64.9.3491-3495.1998 (1998).
Ulloa, O., Canfield, D. E., DeLong, E. F., Letelier, R. M. & Stewart, F. J. Microbial oceanography of anoxic oxygen minimum zones. Proc. Natl. Acad. Sci. 109, 15996–16003. https://doi.org/10.1073/pnas.1205009109 (2012).
Woebken, D. et al. A microdiversity study of anammox bacteria reveals a novel candidatus Scalindua phylotype in marine oxygen minimum zones. Environ. Microbiol. 10, 3106–3119. https://doi.org/10.1111/j.1462-2920.2008.01640.x (2008).
Walsh, D. A. et al. Metagenome of a versatile chemolithoautotroph from expanding oceanic dead zones. Science 326, 578–582. https://doi.org/10.1126/science.1175309 (2009).
Jurado, V. et al. Microbial communities in carbonate precipitates from drip waters in Nerja cave. Spain PeerJ. 10, e13399. https://doi.org/10.7717/peerj.13399 (2022).
Sjöberg, S., Callac, N., Allard, B., Smittenberg, R. H. & Dupraz, C. Microbial communities inhabiting a rare Earth element enriched birnessite-type manganese deposit in the Ytterby Mine, Sweden. Geomicrobiol. J. 35, 657–674. https://doi.org/10.1080/01490451.2017.1399722 (2018).
Brettar, I., Christen, R. & Höfle, M. G. Rheinheimera Baltica gen. nov., sp. nov., a Bluecoloured bacterium isolated from the central Baltic sea. Int. J. Syst. Evol. Microbiol. 52, 1851–1857. https://doi.org/10.1099/00207713-52-5-1851 (2002).
Contos, A. K., James, J. M., Heywood, B., Pitt, K. & Rogers, P. Morphoanalysis of bacterially precipitated subaqueous calcium carbonate from Weebubbie Cave, Australia. Geomicrobiol. J. 18, 331–343. https://doi.org/10.1080/01490450152467822 (2001).
Holmes, A. J. et al. Phylogenetic structure of unusual aquatic microbial formations in nullarbor caves, Australia. Environ. Microbiol. 3, 256–264. https://doi.org/10.1046/j.1462-2920.2001.00187.x (2001).
Strathmann, R. R. Feeding and nonfeeding larval development and life-history evolution in marine invertebrates. Annu. Rev. Ecol. Syst. 16, 339–361 (1985). http://www.jstor.org/stable/2097052 [Accessed 22 Sept. 2025].
Nakagawa, S. et al. Microbiota in the coelomic fluid of two common coastal starfish species and characterization of an abundant Helicobacter-related taxon. Sci. Rep. 7, 1–10. https://doi.org/10.1038/s41598-017-09355-2 (2017).
Quigley, M. S., Santschi, P. H., Hung, C. C., Guo, L. & Honeyman, B. D. Importance of acid polysaccharides for 234Th complexation to marine organic matter. Limnol. Oceanogr. 47, 367–377. https://doi.org/10.4319/lo.2002.47.2.0367 (2002).
Dobson, W. E., Stancyk, S. E., Clements, L. A. & Showman, R. M. Nutrient translocation during early disc regeneration in the Brittlestar Microphiopholis Gracillima (Stimpson) (Echinodermata: Ophiuroidea). Biol. Bull. 180, 167–184. https://doi.org/10.2307/1542439 (1991).
Hoskins, D., Stancyk, S. & Decho, A. Utilization of algal and bacterial extracellular polymeric secretions (EPS) by the deposit-feeding Brittlestar Amphipholis Gracillima (Echinodermata). Mar. Ecol. Prog. Ser. 247, 93–101. https://doi.org/10.3354/meps247093 (2003).
Selck, H., Decho, A. W. & Forbes, V. E. Effects of chronic metal exposure and sediment organic matter on digestive absorption efficiency of cadmium by the deposit-feeding polychaete Capitella species I. Environ. Toxicol. Chem. 18, 1289–1297. https://doi.org/10.1002/etc.5620180631 (1999).
Bhaskar, P. V. & Bhosle, N. B. Bacterial extracellular polymeric substance (EPS): A carrier of heavy metals in the marine food-chain. Environ. Int. 32, 191–198. https://doi.org/10.1016/j.envint.2005.08.010 (2006).
Prabagaran, S. R., Manorama, R., Delille, D. & Shivaji, S. Predominance of Roseobacter, Sulfitobacter, Glaciecola and Psychrobacter in seawater collected off Ushuaia, Argentina, Sub-Antarctica. FEMS Microbiol. Ecol. 59, 342–355. https://doi.org/10.1111/j.1574-6941.2006.00213.x (2007).
Suárez-Moo, P. et al. Characterization of sediment microbial communities at two sites with low hydrocarbon pollution in the Southeast Gulf of Mexico. PeerJ 8, e10339. https://doi.org/10.7717/peerj.10339 (2020).
Cattaneo, A. et al. Carboxylic ester hydrolases from Antarctic psychrophilic psychrobacter strains: from genome prospecting to biotreatment of polyester plastics. Bioresour. Technol. 436, 133052. https://doi.org/10.1016/j.biortech.2025.133052 (2025).
Acknowledgements
We thank Sarah Rubelowsky and Shari Rohret for their support in the field. This work was supported by the project “Tapetes microbianos y microbioma de Asterinides sp., (Echinodermata: Asteroidea) a través de su dinámica y función en la cueva el Aerolito, Isla Cozumel, Quintana Roo, México” (PAPIIT-IN207021, UNAM), the UNAM grant to Vergara-Ovando Cindel; thanks also to the participating institutions and Coco Tortuga A.C. for their additional financial support. G. Medina Tanco acknowledges financial support of DGAPA, through PAPIIT IT102926, and the Institute of Nuclear Sciences ICN-UNAM and OEI DGAJ-DPI-200922-1139. We thank the participation of Ing. Jorge Ramírez Casteñón with Dr. Gustavo Medina-Tanco for development of the CCC (LINX, Institute of Nuclear Sciences, UNAM), Dr. Silvia Espinoza Matías for scanning electron microscopy (Laboratory of Microscopy, Faculty of Sciences, UNAM) and Biol. Mariana Gonzalez-Macedo for technical and bioinformatics support, as well as English revision (Functional Microbial Ecology of Soil and Environmental Protection Group, Faculty of Sciences, UNAM).
Funding
Research funding is a grant provided by Dirección General de Asuntos del Personal Académico, Universidad Nacional Autónoma de México, PAPIIT-IN207021, to financially support the scientific project; and by Universidad Nacional Autónoma de México, to support posgraduate grant.
Author information
Authors and Affiliations
Contributions
F.A.S.M., C.V.O., M.R.O., F.C.G., G.M.T. and N.C. wrote the main manuscript text and C.V.O. prepared Figs. 1 and 2. F.A.S.M., M.R.O. and N.C. developed the protocol experiment and supervised the project. C.V.O. performed laboratory experiments . F.A.S.M. and N.C. supervised the sampling. F.C.G. made the diving sampling. N.C. prepared samples for preservation. M.R.O. supervised experiments in laboratory. G.M.T. developed the CCC (sampling unit). All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.








Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Solís-Marín, F.A., Vergara-Ovando, C., Rojas-Oropeza, M. et al. Asterinides sp. an endemic stygobitic seastar from an anchialine cave and its interactions among prokaryotic communities. Sci Rep (2026). https://doi.org/10.1038/s41598-026-36065-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-36065-5