Abstract
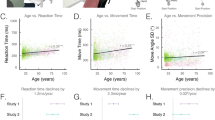
Fine motor function develops into adulthood, but little is known about the differential effects of biological maturation and experience on speed and complex sequential performance of the hand. The objective of the study was to disentangle the differential effects of biological maturation, chronological age, and specific motor experience on fine motor skills of the hand during adolescence. To determine maturity levels, ultrasonic bone age (BA) was assessed in 225 adolescents (123 females; BA mean 13.4 ± 1.5 years, range: 9.9 to 17.9 years). The role of experience was evaluated based on chronological age (CA, mean 13.5 ± 1.2 years, range: 11.1 to 16.5 years), musical instrumental experience, and handedness. When specific musical instrumental experience is not present, biological maturation level is a significant predictor of complex fine motor performance, while chronological age predicts simple repetitive motor performance. When present, the amount of highly specific musical instrumental experience becomes the main predictor of sequential performance.
Similar content being viewed by others
Data availability
The data necessary to reproduce the analyses presented here are not publicly accessible but available from the corresponding author upon reasonable request. The analytic code necessary to reproduce the analyses presented in this paper is publicly accessible. The analyses presented here were not preregistered.
Abbreviations
- BA:
-
Bone age
- CA:
-
Chronological age
- FT:
-
Finger tapping
References
Cech, D. J. & Martin, S. T. Functional Movement Development Across the Life Span (Saunders, 2012).
Vijayakumar, N., de Macks, O., Shirtcliff, Z., Pfeifer, J. H. & E. A. & Puberty and the human brain: insights into adolescent development. Neurosci. Biobehav. Rev. 92, 417–436 (2018).
Gerván, P., Berencsi, A. & Kovacs, I. Vision first? The development of primary visual cortical networks is more rapid than the development of primary motor networks in humans. PLOS ONE. 6, e25572 (2011).
Gerván, P., Soltész, P., Filep, O., Berencsi, A. & Kovács, I. Posterior–anterior brain maturation reflected in perceptual, motor and cognitive performance. Front. Psychol. 8 (2017).
Oldham, S. & Fornito, A. The development of brain network hubs. Dev. Cogn. Neurosci. 36, 100607 (2019).
Váša, F. et al. Conservative and disruptive modes of adolescent change in human brain functional connectivity. Proc. Natl. Acad. Sci. 117, 3248–3253 (2020).
Yeo, S. S., Jang, S. H. & Son, S. M. The different maturation of the corticospinal tract and corticoreticular pathway in normal brain development: Diffusion tensor imaging study. Front. Hum. Neurosci 8 (2014).
Krogman, W. M. Child Growth (University of Michigan Press, 1972).
Itoh, R. & Hirose, N. Relationship among biological maturation, physical characteristics, and motor abilities in youth elite soccer players. J. Strength. Cond. Res. 34, 382 (2020).
Dorfberger, S., Adi-Japha, E. & Karni, A. Sex differences in motor performance and motor learning in children and adolescents: an increasing male advantage in motor learning and consolidation phase gains. Behav. Brain. Res. 198, 165–171 (2009).
Huttenlocher, P. R. Neural Plasticity (Harvard University Press, 2002).
Spear, L. Adolescent neurodevelopment. J. Adolesc. Health. 52, S7–S13 (2013).
Dahms, C., Brodoehl, S., Witte, O. W. & Klingner, C. M. The importance of different learning stages for motor sequence learning after stroke. Hum. Brain. Mapp. 41, 270–286 (2020).
Gao, W. & Lin, W. Frontal parietal control network regulates the anti-correlated default and dorsal attention networks. Hum. Brain. Mapp. 33, 192–202 (2012).
Bartzokis, G. et al. Lifespan trajectory of Myelin integrity and maximum motor speed. Neurobiol. Aging. 31, 1554–1562 (2010).
Betti, S., Castiello, U. & Begliomini, C. Reach-to-Grasp: A multisensory experience. Front. Psychol. 12, 213 (2021).
Heinen, F. et al. Fast corticospinal system and motor performance in children: conduction proceeds skill. Pediatr. Neurol. 19, 217–221 (1998).
Nezua, A. et al. Magnetic stimulation of motor cortex in children: maturity of corticospinal pathway and problem of clinical application. Brain Develop. 19, 176–180 (1997).
Fung, M. H. et al. The development of sensorimotor cortical oscillations is mediated by pubertal testosterone. NeuroImage 264, 119745 (2022).
Vijayakumar, N. et al. A longitudinal analysis of puberty-related cortical development. NeuroImage 228, 117684 (2021).
Herting, M. M. et al. Longitudinal changes in pubertal maturation and white matter microstructure. Psychoneuroendocrinology 81, 70–79 (2017).
Pangelinan, M. M. et al. Puberty and testosterone shape the corticospinal tract during male adolescence. Brain Struct. Funct. 221, 1083–1094 (2016).
Blakemore, S. J., Burnett, S. & Dahl, R. E. The role of puberty in the developing adolescent brain. Hum. Brain. Mapp. 31, 926–933 (2010).
Giedd, J. N. Structural magnetic resonance imaging of the adolescent brain. Ann. N. Y. Acad. Sci. 1021, 77–85 (2004).
Blakemore, S. J. & Choudhury, S. Development of the adolescent brain: implications for executive function and social cognition. J. Child Psychol. Psychiatry. 47, 296–312 (2006).
Juraska, J. M. & Drzewiecki, C. M. Cortical reorganization during adolescence: What the rat can tell Us about the cellular basis. Dev. Cogn. Neurosci. 45, 100857 (2020).
Kovács, I. et al. Ultrasonic bone age fractionates cognitive abilities in adolescence. Sci. Rep. 12, 5311 (2022).
Burnett, S. & Blakemore, S. J. The development of adolescent social cognition. Ann. N. Y. Acad. Sci. 1167, 51–56 (2009).
Gerván, P. et al. Maturation-dependent vulnerability of emotion regulation as a response to COVID-19 related stress in adolescents. J. Pediatr. Nursing Nurs. Care Child. Families. 67, 132–138 (2022).
Desmangles, J. C., Joan, M., Lappe G. L., & Gleb, H. Accuracy of pubertal Tanner staging Self-Reporting. J. Pediatr. Endocrinol. Metab. 19, 213–222 (2006).
Dorn, L. D. & Biro, F. M. Puberty and its measurement: A decade in review. J. Res. Adolescence. 21, 180–195 (2011).
Tanner, J. M. Growth and maturation during adolescence. Nutr. Rev. 39, 43–55 (1981).
Ledford, H. Who exactly counts as an adolescent? Nature 554, 429–431 (2018).
Parent, A. S. et al. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr. Rev. 24, 668–693 (2003).
Roberts, G. J., Parekh, S., Petrie, A. & Lucas, V. S. Dental age assessment (DAA): A simple method for children and emerging adults. Br. Dent. J. 204, E7–E7 (2008).
Worthman, C. M. & Trang, K. Dynamics of body time, social time and life history at adolescence. Nature 554, 451–457 (2018).
Goddings, A., Beltz, A., Peper, J. S., Crone, E. A. & Braams, B. R. Understanding the role of puberty in structural and functional development of the adolescent brain (2019).
Berencsi, A. et al. Musical training improves fine motor function in adolescents. Trends Neurosci. Educ. 27, 100176 (2022).
Szakács, H. et al. Navigating pubertal goldilocks: The optimal Pace for hierarchical brain organization. Adv. Sci. 11, 2308364 (2024).
Civardi, C., Cavalli, A., Naldi, P., Varrasi, C. & Cantello, R. Hemispheric asymmetries of cortico-cortical connections in human hand motor areas. Clin. Neurophysiol. 111, 624–629 (2000).
Herzberg, O., Fletcher, K. K., Schatz, J. L., Adolph, K. E. & Tamis-LeMonda, C. S. Infant exuberant object play at home: Immense amounts of time-distributed, variable practice. Child Dev. 93, 150–164 (2022).
Szűcs, T. & Hejja, E. B. The institutional network and state of music education in Hungary. HERJ Hung. Educ. Res. J. 7, 39–54 (2017).
Herholz, S. C. & Zatorre, R. J. Musical training as a framework for brain plasticity: Behavior, function, and structure. Neuron 76, 486–502 (2012).
Berencsi, A., Gombos, F. & Kovács, I. Capacity to improve fine motor skills in Williams syndrome. J. Intellect. Disabil. Res. 60, 956–968 (2016).
Karni, A. et al. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature 377, 155–158 (1995).
Serrien, D. J. & Sovijärvi-Spapé, M. M. Hemispheric asymmetries and the control of motor sequences. Behav. Brain. Res. 283, 30–36 (2015).
Hervé, P. Y. et al. Handedness, motor skills and maturation of the corticospinal tract in the adolescent brain. Hum. Brain. Mapp. 30, 3151–3162 (2009).
Hammond, G. Correlates of human handedness in primary motor cortex: A review and hypothesis. Neurosci. Biobehavioral Reviews. 26, 285–292 (2002).
Gerván, P. et al. The influence of relative pubertal maturity on executive function development in adolescent girls. Sci. Rep. 4, 28140 (2024).
Utczás, K., Muzsnai, A., Cameron, N., Zsákai, A. & Bodzsár, E. B. A comparison of skeletal maturity assessed by radiological and ultrasonic methods. Am. J. Hum. Biol.. 29, e22966 (2017).
Berencsi, A., Bódizs, R., Gombos, F., László, S., & Kovács, I. Sigma frequency dependent motor learning in Williams syndrome. Sci. Rep. 7, 16759 (2017).
Tonidandel, S. & LeBreton, J. M., RWA. web: A free, comprehensive, web-based, and user-friendly tool for relative weight analyses. J. Bus. Psychol. 30, 207–216 (2015).
Mewton, L. & Squeglia, L. Neurodevelopmental profiles in adolescence: leveraging data from the landmark adolescent brain cognitive development study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 7, 343–345 (2022).
Bethlehem, R. et al. Brain charts for the human lifespan. Nature 604, 525–533 (2022).
Campbell, I. G., Grimm, K. J., de Bie, E. & Feinberg, I. Sex, puberty, and the timing of sleep EEG measured adolescent brain maturation. Proc. National Acad. Sci. 109, 5740–5743 (2012).
Rowley, C. D. et al. Age-related mapping of intracortical Myelin from late adolescence to middle adulthood using T1-weighted MRI. Hum. Brain. Mapp. 38, 3691–3703 (2017).
Gasser, T., Rousson, V., Caflisch, J. & Jenni, O. G. Development of motor speed and associated movements from 5 to 18 years. Dev. Med. Child. Neurol. 52, 256–263 (2010).
Averbeck, B. B. Pruning recurrent neural networks replicates adolescent changes in working memory and reinforcement learning. Proc. Natl. Acad. Sci. U S A. 119, e2121331119 (2022).
Huttenlocher, P. R. Synaptic density in human frontal cortex—Developmental changes and effects of aging. Brain Res. 163, 195–205 (1979).
Rakic, P., Bourgeois, J. P., Eckenhoff, M. F., Zecevic, N. & Goldman-Rakic, P. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science 232, 232–235 (1986).
Delevich, K., Klinger, M., Okada, N. J. & Wilbrecht, L. Coming of age in the frontal cortex: The role of puberty in cortical maturation. Semin. Cell Dev. Biol. 118, 64–72 (2021).
Sakai, J. How synaptic pruning shapes neural wiring during development and, possibly, in disease. Proc. Natl. Acad. Sci. 117, 16096–16099 (2020).
Gaser, C. & Schlaug, G. Brain structures differ between musicians and non-musicians. J. Neurosci. 23, 9240–9245 (2003).
Censor, N., Sagi, D. & Cohen, L. G. Common mechanisms of human perceptual and motor learning. Nat. Rev. Neurosci. 13, 658–664 (2012).
Maruyama, S. et al. Cognitive control affects motor learning through local variations in GABA within the primary motor cortex. Sci. Rep. 11, 18566 (2021).
Korman, M., Raz, N., Flash, T. & Karni, A. Multiple shifts in the representation of a motor sequence during the acquisition of skilled performance. PNAS 100, 12492–12497 (2003).
Karni, A. et al. The acquisition of skilled motor performance: Fast and slow experience-driven changes in primary motor cortex. PNAS 95, 861–868 (1998).
Dow-Edwards, D. et al. Experience during adolescence shapes brain development: From synapses and networks to normal and pathological behavior. Neurotoxicol. Teratol. 76, 106834 (2019).
Yokoi, A. & Diedrichsen, J. Neural organization of hierarchical motor sequence representations in the human neocortex. Neuron 103, 1178–1190e7 (2019).
Schlaug, G., Jäncke, L., Huang, Y., Staiger, J. F. & Steinmetz, H. Increased corpus callosum size in musicians. Neuropsychologia 33, 1047–1055 (1995).
West, K. L. Infant motor development in autism spectrum disorder: A synthesis and meta-analysis. Child Dev. 90, 2053–2070 (2019).
Corbett, B. A. et al. Examination of pubertal timing and tempo in females and males with autism spectrum disorder compared to typically developing youth. Autism Res. 15, 1894–1908 (2022).
Partsch, C. J. et al. Longitudinal evaluation of growth, puberty, and bone maturation in children with Williams syndrome. J. Pediatr. 134, 82–89 (1999).
Tsang, W. W. N., Guo, X., Fong, S. S. M., Mak, K. K. & Pang, M. Y. C. Activity participation intensity is associated with skeletal development in pre-pubertal children with developmental coordination disorder. Res. Dev. Disabil. 33, 1898–1904 (2012).
Katz, N. et al. Bone age in adolescents with schizophrenia and obsessive-compulsive disorder. Schizophr. Res. 33, 119–122 (1998).
Acknowledgements
We thank the generous and amazing parents, adolescents, and schools who participated in this project.The project was supported by the National Research, Development and Innovation Office of Hungary (Grants K-134370 and Advanced 153190 to I.K.), and by the Hungarian Research Network (HUN-REN-ELTE-PPKE Adolescent Development Research Group). It was made in the framework of the PPKE-BTK-KUT-23-1 project, with the support and funding provided by the Faculty of Humanities and Social Sciences of Pázmány Péter Catholic University. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Funding
Open access funding provided by Eötvös Loránd University.
Author information
Authors and Affiliations
Contributions
I.K., A.B., F.G. and P.G. designed the experiment; G.O. recruited and screened participants, K.U. and Z.T. collected and analyzed bone age data, A.B. analyzed the finger tapping data; A.B., K.I. and L.J.F. drafted the paper, L.J.F. prepared the figures with the cooperation of I.K. and A.B., and all authors contributed to discussion and to the text. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Berencsi, A., Gombos, F., Fehér, L.J. et al. The contributions of biological maturity and experience to fine motor development in adolescence. Sci Rep (2026). https://doi.org/10.1038/s41598-026-36220-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-36220-y