Abstract
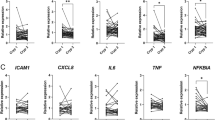
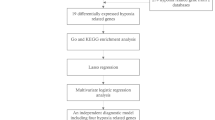
Identifying reliable circulating biomarkers is crucial for improving the diagnosis and risk stratification of patients with ischemic stroke. In this study, we evaluated several whole-blood circulating miRNAs (miR-106b-5p, miR-16-5p, miR-15b-5p, let-7e-5p, and miR-125a-3p/-5p) to determine their diagnostic and disease severity in acute ischemic stroke (AIS). Sixty AIS patients and thirty age- and sex-matched controls were included. Whole-blood miRNAs were quantified at admission and on day 7. Statistical analyses included ROC curves, multivariate logistic regression, and SHAP-based machine learning. Bioinformatic analyses assessed predicted miRNA targets, pathway enrichment, and interaction networks. MiR-125a-3p was significantly reduced in AIS at both time points, while miR-125a-5p was elevated at admission and decreased by day 7. Both miRNAs showed moderate diagnostic value (AUC 0.675 and 0.712, respectively). Higher admission levels of miR-16-5p were strongly associated with greater neurological deficit (NIHSS) and unfavorable outcome (mRS ≥ 3). Multivariate analyses confirmed high miR-16-5p and elevated CRP as independent predictors of poor outcome. Bioinformatic analyses revealed that miR-16-5p targets were enriched in pathways relevant to ischemic injury, including hypoxia response, platelet activation, coagulation, TGF-β and BDNF signaling. A target-interaction network highlighted IL6, FN1, TGFB1, ICAM1, and TLR4 as central nodes linking miR-16-5p to ischemia-inflammatory mechanisms in AIS. Circulating miRNAs display distinct expression patterns in the acute phase of AIS. miR-16-5p emerges as a promising biomarker associated with stroke severity and unfavorable outcome, while miR-125a-3p and miR-125a-5p show potential diagnostic utility. These findings strengthen mechanistic links between platelet-derived miRNAs and ischemic stroke biology. Larger, longitudinal studies integrating functional validation are warranted to confirm their clinical value.
Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Powers, W. J. et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 50, e344–e418 (2019).
Herpich, F. & Rincon, F. Management of acute ischemic stroke. Crit. Care Med. 48, 1654–1663 (2020).
El-Koussy, M., Schroth, G., Brekenfeld, C. & Arnold, M. Imaging of acute ischemic stroke. Eur. Neurol. 72, 309–316 (2014).
Kamtchum-Tatuene, J. & Jickling, G. C. Blood biomarkers for stroke diagnosis and management. Neuromol. Med. 21, 344–368 (2019).
Wang, S.-W., Liu, Z. & Shi, Z.-S. Non-coding RNA in acute ischemic stroke: Mechanisms, biomarkers and therapeutic targets. Cell Transpl. 27, 1763–1777 (2018).
Bartel, D. P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 116, 281–297 (2004).
Ma, H. et al. MicroRNAs in oral lichen planus and potential miRNA–mRNA pathogenesis with essential cytokines: A review.. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 122, 164–173 (2016).
Eyileten, C. et al. MicroRNAs as diagnostic and prognostic biomarkers in ischemic stroke-a comprehensive review and bioinformatic analysis. Cells 7, 249 (2018).
Ge, Q. et al. miRNA in plasma exosome is stable under different storage conditions. Molecules 19, 1568–1575 (2014).
Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007).
Cun, Y. et al. Exosome in crosstalk between inflammation and angiogenesis: A potential therapeutic strategy for stroke. Mediat. Inflamm. 2022, 7006281 (2022).
Sabour, S. The diagnostic value of serum miRNA-221-3p, miRNA-382-5p, and miRNA-4271 in ischemic stroke: Methodological issue to avoid misinterpretation. J. Stroke Cerebrovasc. Dis. 26(5), 1161 (2017).
Chen, Y. et al. Increased circulating exosomal miRNA-223 Is associated with acute ischemic stroke. Front. Neurol. 8, 57 (2017).
Aili, Y. et al. The role of exosomal miRNAs in glioma: Biological function and clinical application. Front. Oncol. 11, 686369 (2021).
Wicik, Z. et al. The role of miRNAs in regulation of platelet activity and related diseases - A bioinformatic analysis. Platelets 33(7), 1052–1064. https://doi.org/10.1080/09537104.2022.2042233 (2022).
Czajka, P. et al. MicroRNA as potential biomarkers of platelet function on antiplatelet therapy: A review. Front. Physiol. 12, 652579 (2021).
Huang, S. et al. Identification of Blood Let-7e-5p as a biomarker for ischemic stroke. PLoS ONE 11, e0163951 (2016).
Tiedt, S. et al. RNA-Seq identifies circulating miR-125a-5p, miR-125b-5p, and miR-143-3p as potential biomarkers for acute ischemic stroke. Circ. Res. 121, 970–980 (2017).
Force, W. T. Recommendations on stroke prevention, diagnosis, and therapy report of the WHO task force on stroke and other cerebrovascular disorders. Stroke 20, 1407–1431 (1989).
Adams, H. P. Jr. et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke 24, 35–41 (1993).
De Rosa, S. et al. Transcoronary concentration gradients of circulating microRNAs in heart failure. Eur. J. Heart Fail. 20, 1000–1010 (2018).
De Rosa, R. et al. Transcoronary concentration gradient of microRNA-133a and outcome in patients with coronary artery disease. Am. J. Cardiol. 120, 15–24 (2017).
Eyileten, C. et al. Alterations in circulating MicroRNAs and the relation of MicroRNAs to maximal oxygen consumption and intima-media thickness in ultra-marathon runners. Int. J. Environ. Res. Public Health 18, 7234 (2021).
Drost, H.-G. & Paszkowski, J. Biomartr: Genomic data retrieval with R. Bioinformatics 33, 1216–1217 (2017).
Piñero, J. et al. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 48, D845–D855 (2019).
Ru, Y. et al. The multiMiR R package and database: Integration of microRNA–target interactions along with their disease and drug associations. Nucleic Acids Res. 42, e133 (2014).
Zofia. .Vignette_wizbionet.Md at Master · Wizbionet/wizbionet. (Github).
Wicik, Z. et al. The crosstalk between bone metabolism, lncRNAs, microRNAs and mRNAs in coronary artery calcification. Genomics 113, 503 (2021).
Doncheva, N. T., Morris, J. H., Gorodkin, J. & Jensen, L. J. Cytoscape StringApp: Network analysis and visualization of proteomics data. J. Proteome Res. 18, 623–632 (2019).
Shannon, P. et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Wu, J., Du, K. & Lu, X. Elevated expressions of serum miR-15a, miR-16, and miR-17-5p are associated with acute ischemic stroke. Int. J. Clin. Exp. Med. 8, 21071–21079 (2015).
Tian, C. et al. Plasma MicroRNA-16 is a biomarker for diagnosis, stratification, and prognosis of hyperacute cerebral infarction. PLoS ONE 11, e0166688 (2016).
Yang, X. et al. MicroRNA-15a/16-1 antagomir ameliorates ischemic brain injury in experimental stroke. Stroke 48, 1941–1947 (2017).
Leung, L. Y. et al. Comparison of miR-124-3p and miR-16 for early diagnosis of hemorrhagic and ischemic stroke. Clin. Chim. Acta 433, 139–144 (2014).
Huang, R. et al. Overexpressing circ_0000831 is sufficient to inhibit neuroinflammation and vertigo in cerebral ischemia through a miR-16-5p-dependent mechanism. Exp. Neurol. 353, 114047 (2022).
Li, Q. et al. Luteoloside attenuates neuroinflammation in focal cerebral ischemia in rats via regulation of the PPARγ/Nrf2/NF-κB signaling pathway. Int. Immunopharmacol. 66, 309–316 (2019).
Chaudhuri, A. D. et al. TNFα and IL-1β modify the miRNA cargo of astrocyte shed extracellular vesicles to regulate neurotrophic signaling in neurons. Cell Death Dis. 9, 363 (2018).
Riccio, A., Ahn, S., Davenport, C. M., Blendy, J. A. & Ginty, D. D. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science 286, 2358–2361 (1999).
Joilin, G. et al. Identification of a potential non-coding RNA biomarker signature for amyotrophic lateral sclerosis. Brain Commun. 2, fcaa053 (2020).
Sandau, U. S. et al. Differential effects of APOE genotype on MicroRNA cargo of cerebrospinal fluid extracellular vesicles in females with Alzheimer’s disease compared to males. Front. Cell Dev. Biol. 10, 864022 (2022).
Norsworthy, P. J. et al. A blood miRNA signature associates with sporadic Creutzfeldt-Jakob disease diagnosis. Nat. Commun. 11, 3960 (2020).
Dewdney, B. et al. Circulating MicroRNAs as biomarkers for acute ischemic stroke: A systematic review. J. Stroke Cerebrovasc. Dis. 27, 522–530 (2018).
Li, P. et al. An Antagomir to MicroRNA-106b-5p ameliorates cerebral ischemia and reperfusion injury in rats via inhibiting apoptosis and oxidative stress. Mol. Neurobiol. 54, 2901–2921 (2017).
Reijerkerk, A. et al. MicroRNAs regulate human brain endothelial cell-barrier function in inflammation: Implications for multiple sclerosis. J. Neurosci. 33, 6857–6863 (2013).
Muramatsu, F., Kidoya, H., Naito, H., Sakimoto, S. & Takakura, N. microRNA-125b inhibits tube formation of blood vessels through translational suppression of VE-cadherin. Oncogene 32, 414–421 (2013).
Song, B., Xu, J., Zhong, P. & Fang, L. MiR-125a-5p silencing inhibits cerebral ischemia-induced injury through targeting IGFBP3. Folia Neuropathol. 59, 121–130 (2021).
Zhao, Y. Y., Wang, W. A. & Hu, H. Treatment with recombinant tissue plasminogen activator alters the microRNA expression profiles in mouse brain after acute ischemic stroke. Neurol. Sci. 36, 1463–1470 (2015).
Liu, R., Luo, S., Zhang, Y. S. & Tsang, C. K. Plasma metabolomic profiling of patients with transient ischemic attack reveals positive role of neutrophils in ischemic tolerance. EBioMedicine 97, 104845 (2023).
Karam, R. A., Amer, M. M. & Zidan, H. E. Long noncoding RNA NEAT1 expression and its target miR-124 in diabetic ischemic stroke patients. Genet. Test. Mol. Biomarkers 26, 398–407 (2022).
Zhou, X. & Qi, L. miR-124 Is downregulated in serum of acute cerebral infarct patients and shows diagnostic and prognostic value. Clin. Appl. Thromb. Hemost. 27, 10760296211035446 (2021).
Bombaci, A. et al. Early-phase fluid diagnostic biomarkers in acute ischemic stroke: An umbrella review. medRxiv (2025) https://doi.org/10.1101/2025.07.09.25331017.
Popa, D.-I. et al. Performance of GFAP and UCH-L1 for early acute stroke diagnosis in the emergency department. J. Clin. Med. 14, 4746 (2025).
Rashidian, J. et al. Cell cycle machinery and stroke. Biochimica et Biophysica Acta (BBA) – Mol. Basis Dis. 1772, 484–493 (2007).
Becker, E. B. E. & Bonni, A. Beyond proliferation–cell cycle control of neuronal survival and differentiation in the developing mammalian brain. Semin. Cell Dev. Biol. 16, 439–448 (2005).
Hua, Y., Zhang, W., Xie, Z., Xu, N. & Lu, Y. MMP-2 is mainly expressed in arterioles and contributes to cerebral vascular remodeling associated with TGF-β1 signaling. J. Mol. Neurosci. 59, 317–325 (2016).
Bobik, A. Transforming growth factor-betas and vascular disorders. Arterioscler. Thromb. Vasc. Biol. 26, 1712–1720 (2006).
Gao, H., Yang, L. & Shao, Y. SIRT1 / NF-κB pathway on neuronal apoptosis in rats with ischemic stroke. Cell. Mol. Biol. 68, 77–82 (2022).
Bruna, B. et al. The signaling pathways underlying BDNF-induced Nrf2 hippocampal nuclear translocation involve ROS, RyR-Mediated Ca signals, ERK and PI3K. Biochem. Biophys. Res. Commun. 505, 201–207 (2018).
Farina, M., Vieira, L. E., Buttari, B., Profumo, E. & Saso, L. The Nrf2 pathway in ischemic stroke: A review. Molecules 26, 5001 (2021).
Le Blanc, J. et al. Platelets selectively regulate the release of BDNF, but not that of its precursor protein, proBDNF. Front. Immunol. 11, 575607 (2020).
Want, A., Nan, X., Kokkali, E., Barde, Y. A. & Morgan, J. E. Brain-derived neurotrophic factor released from blood platelets prevents dendritic atrophy of lesioned adult central nervous system neurons. Brain Commun. 5, fcad046 (2023).
Chen, A. I., Xiong, L.-J., Tong, Y. U. & Mao, M. The neuroprotective roles of BDNF in hypoxic ischemic brain injury. Biomed. Rep. 1, 167 (2012).
Mourão, A. M. et al. Plasma levels of brain-derived neurotrophic factor are associated with prognosis in the acute phase of ischemic stroke. J. Stroke Cerebrovasc. Dis. 28, 735–740 (2019).
Maisonpierre, P. C. et al. Human and rat brain-derived neurotrophic factor and neurotrophin-3: Gene structures, distributions, and chromosomal localizations. Genomics 10, 558–568 (1991).
Zhu, H. et al. Interleukins and ischemic stroke. Front. Immunol. 13, 828447 (2022).
Armstead, W. M. et al. Retraction note: Release of IL-6 after stroke contributes to impaired cerebral autoregulation and hippocampal neuronal necrosis through NMDA receptor activation and upregulation of ET-1 and JNK. Transl. Stroke Res. https://doi.org/10.1007/s12975-024-01264-7 (2024).
Kerkis, I., da Silva, Á. P. & Araldi, R. P. The impact of interleukin-6 (IL-6) and mesenchymal stem cell-derived IL-6 on neurological conditions. Front. Immunol. 15, 1400533 (2024).
Webb, C. E., Vautrinot, J. & Hers, I. IL-6 as a mediator of platelet hyper-responsiveness. Cells 14, 766 (2025).
Pawluk, H. et al. The role of IL-6 in ischemic stroke. Biomolecules 15, 470 (2025).
Ot, S., Zafar, L., Beg, M. & Siddiqui, O. A. Association of mean platelet volume with risk factors and functional outcome in acute ischemic stroke. J. Neurosci. Rural Pract. 12, 764–769 (2021).
Cramer, S. C. An overview of therapies to promote repair of the brain after stroke. Head Neck 33(Suppl 1), S5-7 (2011).
Bhattarai, P. et al. Rare genetic variation in fibronectin 1 (FN1) protects against APOEε4 in Alzheimer’s disease. Acta Neuropathol. 147, 1–20 (2024).
Phillips, C. M., Stamatovic, S. M., Keep, R. F. & Andjelkovic, A. V. Epigenetics and stroke: role of DNA methylation and effect of aging on blood–brain barrier recovery. Fluids Barriers CNS 20, 14 (2023).
Howe, M. D. et al. Fibronectin induces the perivascular deposition of cerebrospinal fluid-derived amyloid-β in aging and after stroke. Neurobiol. Aging 72, 1 (2018).
Dhanesha, N. et al. Fn-EDA (Fibronectin Containing Extra Domain A) in the Plasma, but not endothelial cells, exacerbates stroke outcome by promoting thrombo-inflammation. Stroke 50, 1201–1209 (2019).
Prakash, P., Kulkarni, P. P., Lentz, S. R. & Chauhan, A. K. Cellular fibronectin containing extra domain A promotes arterial thrombosis in mice through platelet Toll-like receptor 4. Blood 125, 3164–3172 (2015).
Maurer, E. et al. Fibrillar cellular fibronectin supports efficient platelet aggregation and procoagulant activity. Thromb. Haemost. 114, 1175–1188 (2015).
American Heart Association Journals. American Heart Association Journals https://www.ahajournals.org/doi/abs/https://doi.org/10.1161/JAHA.125.044299.
Zhai, Z. et al. Fibrinogen controls human platelet fibronectin internalization and cell-surface retention. J. Thromb. Haemost. 5, 1740–1746 (2007).
Mosher, D. F. Adding complexity to fibronectin-platelet interactions. Arterioscler. Thromb. Vasc. Biol. 28, 203–204 (2008).
Krupinski, J., Kumar, P., Kumar, S. & Kaluza, J. Increased expression of TGF-beta 1 in brain tissue after ischemic stroke in humans. Stroke 27, 852 (1996).
Kim, Y. & Lee, C. The gene encoding transforming growth factor β1 confers risk of ischemic stroke and vascular dementia. Stroke https://doi.org/10.1161/01.STR.0000244782.76917.87 (2006).
Moustakas, A. & Miyazawa, K. TGF-β in Human Disease. (Springer Science & Business Media, 2013).
Popek, M., Bobula, B., Orzeł-Gajowik, K. & Zielińska, M. The effect of TGF-β1 reduced functionality on the expression of selected synaptic proteins and electrophysiological parameters: Implications of changes observed in acute hepatic encephalopathy. Int. J. Mol. Sci. 23, 1081 (2022).
Hewitt, B. J., Ali, M., Hubbard, J., Hill, L. J. & Botfield, H. Systematic review of the differential effects of TGF-β1 in ischemic and hemorrhagic preclinical stroke models. J. Am. Heart Assoc. 14, e037890 (2025).
Zhang, L. et al. Extracellular vesicles from hypoxia-preconditioned microglia promote angiogenesis and repress apoptosis in stroke mice via the TGF-β/Smad2/3 pathway. Cell Death Dis. 12, 1068 (2021).
Li, F., Kang, X., Xin, W. & Li, X. The emerging role of extracellular vesicle derived from neurons/neurogliocytes in central nervous system diseases: Novel insights into ischemic stroke. Front. Pharmacol. 13, 890698 (2022).
Serralheiro, P., Soares, A., Costa Almeida, C. M. & Verde, I. TGF-β1 in vascular wall pathology: Unraveling chronic venous insufficiency pathophysiology. Int. J. Mol. Sci. 18, 2534 (2017).
Spakova, T., Janockova, J. & Rosocha, J. Characterization and therapeutic use of extracellular vesicles derived from platelets. Int. J. Mol. Sci. 22, 9701 (2021).
Yan, J. et al. Platelet-derived microvesicles promote endothelial progenitor cell proliferation in intimal injury by delivering TGF-β1. FEBS J. 287, 5196–5217 (2020).
Wang, L., Chen, Y., Feng, D. & Wang, X. Serum ICAM-1 as a predictor of prognosis in patients with acute ischemic stroke. Biomed. Res. Int. 2021, 5539304 (2021).
Stegner, D., Klaus, V. & Nieswandt, B. Platelets as modulators of cerebral ischemia/reperfusion injury. Front. Immunol. 10, 488721 (2019).
Couch, Y. Differential effects of ischemia and inflammation on plasma-derived extracellular vesicle characteristics and function. (2025) https://doi.org/10.21203/rs.3.rs-6937248/v1
Yin, P., Wei, Y., Wang, X., Zhu, M. & Feng, J. Roles of specialized pro-resolving lipid mediators in cerebral ischemia reperfusion injury. Front. Neurol. 9, 378492 (2018).
Li, C. et al. Cerebral endothelial cell-derived small extracellular vesicles enhance neurovascular function and neurological recovery in rat acute ischemic stroke models of mechanical thrombectomy and embolic stroke treatment with tPA. J. Cereb. Blood Flow Metab. https://doi.org/10.1177/0271678X21992980 (2021).
Gesuete, R., Kohama, S. G. & Stenzel-Poore, M. Toll-like receptors and ischemic brain injury. J. Neuropathol. Exp. Neurol. 73, 378 (2014).
Shichita, T. et al. Novel therapeutic strategies targeting innate immune responses and early inflammation after stroke. J. Neurochem. 123(Suppl 2), 29–38 (2012).
Rao, D., Sang, C., Lai, Z., Zhong, J. & Tang, Z. Roles of extracellular vesicles in cerebral protection of ischemic stroke. Neuro Endocrinol. Lett. 42, 160 (2021).
Roseborough, A. D. et al. Plasma derived extracellular vesicle biomarkers of microglia activation in an experimental stroke model. J. Neuroinflam. 20, 20 (2023).
Qiu, L. et al. Mesenchymal stem cell-derived extracellular vesicles attenuate tPA-induced blood-brain barrier disruption in murine ischemic stroke models. Acta Biomater. 154, 424–442 (2022).
Ding, N. et al. Toll-like receptor 4 regulates platelet function and contributes to coagulation abnormality and organ injury in hemorrhagic shock and resuscitation. Circ. Cardiovasc. Genet. 7, 615–624 (2014).
de Rezende, V. L. et al. The role of extracellular vesicles in brain-peripheral communication following ischemic stroke: Implications for neural repair and regeneration. J. Neurochem. 169, e70155 (2025).
Weinstein, J. R. et al. Functional polymorphisms in toll-like receptor 4 are associated with worse outcome in acute ischemic stroke patients. NeuroReport 25, 580 (2014).
Sun, M. et al. Isoflurane preconditioning provides neuroprotection against stroke by regulating the expression of the TLR4 signalling pathway to alleviate microglial activation. Sci. Rep. 5, 11445 (2015).
Suzuki, Y. et al. Pharmacological inhibition of TLR4-NOX4 signal protects against neuronal death in transient focal ischemia. Sci. Rep. 2, 896 (2012).
Krug, T. et al. TTC7B emerges as a novel risk factor for ischemic stroke through the convergence of several genome-wide approaches. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 32, 1061 (2012).
Fjdm, M. et al. Transcriptomic Analysis of Extracellular Vesicles in the Search for Novel Plasma and Thrombus Biomarkers of Ischemic Stroke Etiologies. Int. J. Mol. Sci. 25, 4379 (2024).
Kanki, H. et al. Importance of microRNAs by mRNA-microRNA integration analysis in acute ischemic stroke patients. J. Stroke Cerebrovasc. Dis.: Off. J. Natl. Stroke Assoc. 32(9), 107277 (2023).
Acknowledgements
This work was written by the members (MP, DMG, CE, ZW) of the International and Intercontinental Cardiovascular and Cardiometabolic Research Team (I-COMET; www.icomet.science).
Funding
MP was supported financially as part of the research grant ‘OPUS’ from National Science Center, Poland (grant number 2018/31/B/NZ7/01137) and Medical University of Warsaw (grant number 1M9/2/M/MBM/N/21).
Author information
Authors and Affiliations
Contributions
Writing—original draft preparation, CE, PC, ZW, MP; writing—review and editing, CE, PC, ZW, AS, DMG, AC, MP, ID, AWC, SA; bioinformatic analysis– ZW, visualization, CE, ZW; supervision, CE, DMG, MP; laboratory and computer analysis, CE, SA; Data preparation; CE, ID, AWC. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Authors declare no conflict of interest related to this work.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Medical University of Warsaw, Warsaw, Poland (protocol code KB/148/2017, date of approval: 4 July 2017).
Informed consent
Informed consent was obtained from all subjects involved in the study and written informed consent has been obtained from the patient(s) to publish this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Eyileten, C., Wicik, Z., Shahzadi, A. et al. An integrative clinical and bioinformatic analysis identifies MicroRNAs as biomarkers of ischemic stroke severity. Sci Rep (2026). https://doi.org/10.1038/s41598-026-36494-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-36494-2