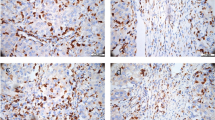
Abstract
In the era of precision medicine, the tumor microenvironment (TME) of esophageal cancer needs further insight, to identify unfavorable biology leading to poor outcomes. This study analyzed the TME of esophageal cancer in relation to clinicopathologic parameters, and neoadjuvant treatment. A series of patients operated for esophageal cancer with curative intent between 01.2009 and 12.2021 were included. Initial biopsies and surgical specimens of all patients underwent immunohistochemical analysis, to detect the immune infiltrate markers CD3+, CD8, CD163, CD68, PD-L1 and FoxP3+. The CPS score was used for PD-L1 quantification, whereas the Mandard regression grade (TRG) assessed pathologic response to neoadjuvant treatment (NAT). Continuous variables were compared with the Mann-Whitney-U test, and categorical ones with the Chi-2 test; paired-data tests were used when appropriate. Significance threshold was set at p < 0.05. Overall, 68 patients (82.4% males, mean age 62.4±9.4 years, 79.4% adenocarcinoma) were included. TME in smokers had lower M2-like (CD163+, p = 0.010) and total macrophages (CD68+, p = 0.001), but similar CD163/68 ratio and T-cells as non-smokers. Squamous cell cancer compared to adenocarcinoma showed lower M2-like macrophages (p = 0.023) and T-cell infiltration (p = 0.006). NAT increased macrophages in the TME, while depleting Treg/FoxP3 + cells. Poor responders to NAT had similar baseline TME characteristics as complete responders, but they displayed higher total macrophage count (CD68+) after NAT (p = 0.026). In the present series, the TME of active smokers and patients with squamous cell cancer had a significantly reduced M2-like macrophage infiltration. Neoadjuvant treatment recruited macrophages and T-cells in the TME, but interestingly, an increased macrophage count upon final histology was related to poor response to treatment. The present study provides valuable insight to the TME composition of esophageal cancer and its modification after NAT, however, further studies are needed to assess the exact functional role of TME elements, and their impact on clinical outcomes.
Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Yang, H., Wang, F., Hallemeier, C. L., Lerut, T. & Fu, J. Oesophageal cancer. Lancet 404(10466), 1991–2005 (2024).
Lonie, J. M., Barbour, A. P. & Dolcetti, R. Understanding the immuno-biology of oesophageal adenocarcinoma: towards improved therapeutic approaches. Cancer Treat. Rev. 98, 102219 (2021).
Al-Batran, S. E. et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 393(10184), 1948–1957 (2019).
Shapiro, J. et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 16(9), 1090–1098 (2015).
van Hagen, P. et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl. J. Med. 366(22), 2074–2084 (2012).
Mantziari, S., Allemann, P., Winiker, M., Demartines, N. & Schafer, M. Locoregional tumor extension and preoperative smoking are significant risk factors for early recurrence after esophagectomy for cancer. World J. Surg. 42(7), 2209–2217 (2018).
Kelly, R. J. et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl. J. Med. 384(13), 1191–1203 (2021).
Janjigian, Y. Y. et al. Perioperative durvalumab in gastric and gastroesophageal junction cancer. N Engl. J. Med. 393(3), 217–230 (2025).
Lorenzen, S. et al. Perioperative atezolizumab plus fluorouracil, leucovorin, oxaliplatin, and docetaxel for resectable esophagogastric cancer: interim results from the randomized, multicenter, phase II/III DANTE/IKF-s633 trial. J. Clin. Oncol. 42(4), 410–420 (2024).
Belle, C. J., Lonie, J. M., Brosda, S. & Barbour, A. P. Tumour microenvironment influences response to treatment in oesophageal adenocarcinoma. Front. Immunol. 14, 1330635 (2023).
Donlon, N. E. et al. Impact of radiotherapy on the immune landscape in oesophageal adenocarcinoma. World J. Gastroenterol. 28(21), 2302–2319 (2022).
Davern, M. et al. The tumour immune microenvironment in oesophageal cancer. Br. J. Cancer. 125(4), 479–494 (2021).
Tian, X. et al. Identification of tumor-infiltrating immune cells and prognostic validation of tumor-infiltrating mast cells in adrenocortical carcinoma: results from bioinformatics and real-world data. Oncoimmunology 9(1), 1784529 (2020).
Pittet, M. J., Michielin, O. & Migliorini, D. Clinical relevance of tumour-associated macrophages. Nat. Rev. Clin. Oncol. 19(6), 402–421 (2022).
Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, D. K. & AWMF). : Diagnostik und Therapie der Plattenepithelkarzinome und Adenokarzinome des Ösophagus, Langversion 4.0, 2023, AWMF-Registernummer: 021-023OL https://www.leitlinienprogramm-onkologie.de/leitlinien/oesophaguskarzinom/; Zugriff am (2025).
Mandard, A. M. et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 73(11), 2680–2686 (1994).
Grillo, F., Fassan, M., Sarocchi, F., Fiocca, R. & Mastracci, L. HER2 heterogeneity in gastric/gastroesophageal cancers: from benchside to practice. World J. Gastroenterol. 22(26), 5879–5887 (2016).
Ieni, A. et al. Discordance rate of HER2 status in primary gastric carcinomas and synchronous lymph node metastases: a multicenter retrospective analysis. Int. J. Mol. Sci. 15(12), 22331–22341 (2014).
Seo, S. et al. Loss of HER2 positivity after anti-HER2 chemotherapy in HER2-positive gastric cancer patients: results of the gastric cancer HER2 reassessment study 3 (GASTHER3). Gastric Cancer. 22(3), 527–535 (2019).
Svenningsen, C. PD-L1 IHC 22C3 pharmdx interpretation Manual, esophageal cancer. Esophageal Cancer (2023).
Janjigian, Y. Y. et al. First-Line nivolumab plus chemotherapy for advanced gastric, gastroesophageal Junction, and esophageal adenocarcinoma: 3-year follow-up of the phase III checkmate 649 trial. J. Clin. Oncol. 42(17), 2012–2020 (2024).
Formica, V. et al. PD-L1 thresholds predict efficacy of immune checkpoint Inhibition in first-line treatment of advanced gastroesophageal adenocarcinoma. A systematic review and meta-analysis of seven phase III randomized trials. ESMO Open. 9(11), 103967 (2024).
Moehler, M. et al. Recent progress and current challenges of immunotherapy in advanced/metastatic esophagogastric adenocarcinoma. Eur. J. Cancer. 176, 13–29 (2022).
Park, Y. et al. PD-L1 testing in gastric cancer by the combined positive score of the 22C3 pharmdx and SP263 assay with clinically relevant cut-offs. Cancer Res. Treat. 52(3), 661–670 (2020).
Hofmann, M. et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 52(7), 797–805 (2008).
Bartley, A. N. et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: guideline from the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J. Clin. Oncol. 35(4), 446–464 (2017).
Dos Santos Cunha, A. C. et al. Dissecting the inflammatory tumor microenvironment of esophageal adenocarcinoma: mast cells and natural killer cells are favorable prognostic factors and associated with less extensive disease. J. Cancer Res. Clin. Oncol. 149(10), 6917–6929 (2023).
Noble, F. et al. Tumour infiltrating lymphocytes correlate with improved survival in patients with oesophageal adenocarcinoma. Cancer Immunol. Immunother. 65(6), 651–662 (2016).
Schoemmel, M. et al. Distribution of tumor-infiltrating-T-lymphocytes and possible tumor-escape mechanisms avoiding immune cell attack in locally advanced adenocarcinomas of the esophagus. Clin. Transl Oncol. 23(8), 1601–1610 (2021).
Gao, Y. et al. Prognostic value of tumor-infiltrating lymphocytes in esophageal cancer: an updated meta-analysis of 30 studies with 5,122 patients. Ann. Transl Med. 8(13), 822 (2020).
Soeratram, T. T. et al. Tumor-immune landscape patterns before and after chemoradiation in resectable esophageal adenocarcinomas. J. Pathol. 256(3), 282–296 (2022).
Yang, D. C. & Chen, C. H. Cigarette Smoking-Mediated macrophage reprogramming: mechanistic insights and therapeutic implications. J. Nat. Sci. 4(11) (2018).
Zhou, L., Zhao, T., Zhang, R., Chen, C. & Li, J. New insights into the role of macrophages in cancer immunotherapy. Front. Immunol. 15, 1381225 (2024).
Sun, Y. et al. The effect of smoking on the immune microenvironment and immunogenicity and its relationship with the prognosis of immune checkpoint inhibitors in non-small cell lung cancer. Front. Cell. Dev. Biol. 9, 745859 (2021).
Lou, Y. S. et al. Chemotherapy elevates cell surface PD-L1 and MHC-I expression in apoptotic gastric cancer cells. Int. J. Immunopathol. Pharmacol. 39, 3946320251338662 (2025).
Zhao, S. et al. Molecular profiles of different PD-L1 expression in patients with esophageal squamous cell carcinoma. Cancer Biol. Ther. 24(1), 2256927 (2023).
Chen, W. C. et al. Change in PD-L1 and CD8 expression after chemoradiotherapy for esophageal squamous cell carcinoma. Biomedicines. 10(8) (2022).
Kim, Y., Yamamoto, S. & Kato, K. Profile of nivolumab in the treatment of resected esophageal squamous cell carcinoma: A review of the clinical data. Cancer Manag Res. 15, 399–406 (2023).
Guo, L. et al. Variation of programmed death ligand 1 expression after platinum-based neoadjuvant chemotherapy in lung cancer. J. Immunother. 42(6), 215–220 (2019).
Herter, J. M. et al. Influence of chemoradiation on the immune microenvironment of cervical cancer patients. Strahlenther Onkol. 199(2), 121–130 (2023).
Zhang, J. et al. Tumor associated macrophages in esophageal squamous carcinoma: promising therapeutic implications. Biomed. Pharmacother. 167, 115610 (2023).
Chen, J. et al. Tumor-associated macrophage (TAM)-secreted CCL22 confers cisplatin resistance of esophageal squamous cell carcinoma (ESCC) cells via regulating the activity of diacylglycerol kinase alpha (DGKalpha)/NOX4 axis. Drug Resist. Updat. 73, 101055 (2024).
Escamilla, J. et al. CSF1 receptor targeting in prostate cancer reverses macrophage-mediated resistance to androgen blockade therapy. Cancer Res. 75(6), 950–962 (2015).
Omstead, A. N. et al. CSF-1R inhibitor, pexidartinib, sensitizes esophageal adenocarcinoma to PD-1 immune checkpoint blockade in a rat model. Carcinogenesis 43(9), 842–850 (2022).
Geng, Z. et al. Integrative analyses of bulk and single-cell RNA-seq reveals the correlation between SPP1(+) macrophages and resistance to neoadjuvant chemoimmunotherapy in esophageal squamous cell carcinoma. Cancer Immunol. Immunother. 73(12), 257 (2024).
Funding
This work was supported by a research grant from the Foundation for the University of Lausanne and the Chuard-Schmidt Foundation, Lausanne, Switzerland.
Author information
Authors and Affiliations
Contributions
FF and HTF contributed equally to study conception, data collection, statistical analysis, and drafting of the manuscript. CS provided pathology expertise and performed histological review. SW and NP assisted with immunohistochemistry and data acquisition. DF and MS contributed to interpretation of results, and critical revision of the manuscript. SM conceived and designed the study, secured funding, supervised all stages of the project, coordinated the multidisciplinary collaboration, and had overall responsibility for data interpretation and manuscript preparation.All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the local Ethics Committee of the Canton of Vaud, Switzerland (CER-VD No. 2022 − 01747, OesoMET). Written informed consent for the reuse of clinical data and histologic specimens was obtained from all patients.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fasquelle, F., Teixeira Farinha, H., Sempoux, C. et al. The tumor microenvironment in esophageal cancer and its association with clinical features and neoadjuvant treatment response. Sci Rep (2026). https://doi.org/10.1038/s41598-026-36537-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-36537-8