Abstract
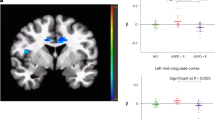
Behavioural, structural, and functional neuroimaging differences exist between individuals with antisocial personality disorder with (ASPD + P) or without psychopathy (ASPD-P). However, the aetiological mechanisms underpinning such differences remain unclear, hindering treatment development. Intranasal oxytocin (OT) has shown modulatory effects on social brain function in healthy and antisocial populations. We investigated the effects of OT on resting-state brain function in individuals with violent offending histories with ASPD+/-P using arterial spin labelling to measure regional cerebral blood flow (rCBF). A double-blind, placebo-controlled, crossover design was employed with males with ASPD (ASPD + P: N = 17, ASPD-P: N = 14) and healthy male non-offenders (N = 22). Both ASPD subtypes exhibited reduced rCBF in frontotemporal regions compared to non-offenders. Individuals with ASPD + P showed significantly greater rCBF increases in posterior default mode network regions compared to individuals with ASPD-P. OT administration selectively decreased rCBF in the left basal ganglia of the ASPD-P group, an effect not observed in ASPD + P or non-offender groups. These findings highlight functional brain differences between individuals with ASPD + P and ASPD-P at rest and demonstrate oxytocin’s differential impact on resting-state measures. Further understanding of the origins of these neurobiological differences could inform targeted therapeutic strategies for individuals with ASPD with and without psychopathy.
Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
First, M. B., Williams, J. B. W., Benjamin, L. S. & Spitzer, R. L. User’s Guide for the SCID-5-PD (Structured Clinical Interview for DSM-5 Personality Disorder) (American Psychiatric Association, 2015).
De Brito, S. A. et al. Psychopathy. Nat. Rev. Dis. Primers. 7, 1–21 (2021).
Hare, R. D. Manual for the Hare Psychopathy Checklist-Revised (Guilford, 1991). https://doi.org/10.1007/978-0-387-79948-3_837
Moffitt, T. E. Male antisocial behaviour in adolescence and beyond. Nat. Hum. Behav. 2, 177–186 (2018).
Azevedo, J., Vieira-Coelho, M., Castelo-Branco, M., Coelho, R. & Figueiredo-Braga, M. Impulsive and premeditated aggression in male offenders with antisocial personality disorder. PLoS One. 15, e0229876 (2020).
Riser, R. E. & Kosson, D. S. Criminal behavior and cognitive processing in male offenders with antisocial personality disorder with and without comorbid psychopathy. Personality Disorders: Theory Res. Treat. 4, 332–340 (2013).
Olver, M. E., Lewis, K. & Wong, S. C. P. Risk reduction treatment of High-Risk psychopathic offenders: the relationship of psychopathy and treatment change to violent recidivism. Personality Disorders: Theory Res. Treat. 4, 160–167 (2013).
Kosson, D. S., Lorenz, A. R. & Newman, J. P. Effects of comorbid psychopathy on criminal offending and emotion processing in male offenders with antisocial personality disorder. J. Abnorm. Psychol. 115, 798–806 (2006).
Mayer, S. V., Jusyte, A., Klimecki-Lenz, O. M. & Schönenberg, M. Empathy and altruistic behavior in antisocial violent offenders with psychopathic traits. Psychiatry Res. 269, 625–632 (2018).
Shepherd, S. M., Campbell, R. E., Ogloff, J. R. P. & Psychopathy Antisocial personality Disorder, and reconviction in an Australian sample of forensic patients. Int. J. Offender Ther. Comp. Criminol. 62, 609–628 (2018).
Dugré, J. R. et al. Neurofunctional abnormalities in antisocial spectrum: A meta-analysis of fMRI studies on five distinct neurocognitive research domains. Neurosci. Biobehav Rev. 119, 168–183 (2020).
De Brito, S. A., Viding, E., Kumari, V., Blackwood, N. & Hodgins, S. Cool and hot executive function impairments in violent offenders with antisocial personality disorder with and without psychopathy. PLoS One. 8, e65566 (2013).
Tully, J. et al. Impaired striatal glutamate/GABA regulation in violent offenders with antisocial personality disorder and psychopathy. Mol. Psychiatry. 2024, 1–9. https://doi.org/10.1038/s41380-024-02437-4 (2024).
Gregory, S. et al. The antisocial brain: psychopathy matters. A structural MRI investigation of antisocial male violent offenders. Arch. Gen. Psychiatry. 69, 962–972 (2012).
Gregory, S. et al. Punishment and psychopathy: a case-control functional MRI investigation of reinforcement learning in violent antisocial personality disordered men. Lancet Psychiatry. 2, 153–160 (2015).
Decety, J., Skelly, L. R. & Kiehl, K. A. Brain response to empathy-eliciting scenarios involving pain in incarcerated individuals with psychopathy. JAMA Psychiatry. 70, 638–645 (2013).
Tully, J. et al. Oxytocin normalises the implicit processing of fearful faces in psychopathy: a randomised crossover study using fMRI. Nat. Mental Health. 1, 420–427 (2023).
Finn, E. S. Is it time to put rest to rest? Trends Cogn. Sci. 25, 1021–1032 (2021).
Fox, M. D. & Greicius, M. Clinical applications of resting state functional connectivity. Front. Syst. Neurosci. 4, 1–13 (2010).
Menon, V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 15, 483–506 (2011).
Gusnard, D. A. & Raichle, M. E. Searching for a baseline: functional imaging and the resting human brain. Nat. Rev. Neurosci. 2, 685–694 (2001).
Moutoussis, M. et al. Decision-making ability, psychopathology, and brain connectivity. Neuron 109, 2025–2040 (2021).
Tavor, I. et al. Task-free MRI predicts individual differences in brain activity during task performance. Sci. (1979). 352, 216–220 (2016).
Molloy, M. F. et al. Regional, but not brain-wide, graph theoretic measures are robustly and reproducibly linked to general cognitive ability. Cereb. Cortex 35 (2025).
Espinoza, F. A. et al. Aberrant functional network connectivity in psychopathy from a large (N = 985) forensic sample. Hum. Brain Mapp. 39, 2624–2634 (2018).
Contreras-Rodríguez, O. et al. Functional connectivity bias in the prefrontal cortex of psychopaths. Biol. Psychiatry. 78, 647–655 (2015).
Tillem, S. et al. Psychopathy is associated with shifts in the organization of neural networks in a large incarcerated male sample. Neuroimage Clin. 24, 1–12 (2019).
Jiang, W. et al. Disrupted functional connectome in antisocial personality disorder. Brain Imaging Behav. 11, 1071–1084 (2017).
Tang, Y., Jiang, W., Liao, J., Wang, W. & Luo, A. Identifying individuals with antisocial personality disorder using Resting-State fMRI. PLoS One. 8, e60652 (2013).
Simon, A. B. & Buxton, R. B. Understanding the dynamic relationship between cerebral blood flow and the BOLD signal: implications for quantitative functional MRI. Neuroimage 116, 158–167 (2015).
Stewart, S. B., Koller, J. M., Campbell, M. C. & Black, K. J. Arterial spin labeling versus BOLD in direct challenge and drug-task interaction Pharmacological fMRI. PeerJ 2, 1–14 (2014).
Kolla, N. J. & Houle, S. Single-Photon emission computed tomography and positron emission tomography studies of antisocial personality disorder and aggression: a targeted review. Curr. Psychiatry Rep. 21, 1–11 (2019).
Soderstrom, H., Tullberg, M., Wikkelsö, C., Ekholm, S. & Forsman, A. Reduced regional cerebral blood flow in non-psychotic violent offenders. Psychiatry Res. Neuroimaging. 98, 29–41 (2000).
Alia-Klein, N. et al. Reactions to media violence: it’s in the brain of the beholder. PLoS One. 9, e107260 (2014).
Borogovac, A. & Asllani, I. Arterial spin labeling (ASL) fMRI: Advantages, theoretical constrains and experimental challenges in neuroscience. Int J Biomed Imaging 2012, 1–13 (2012).
Hodkinson, D. J. et al. Quantifying the test–retest reliability of cerebral blood flow measurements in a clinical model of on-going post-surgical pain: A study using pseudo-continuous arterial spin labelling. Neuroimage Clin. 3, 301–310 (2013).
Menon, R. & Neumann, I. D. Detection, processing and reinforcement of social cues: regulation by the Oxytocin system. Nat. Rev. Neurosci. 24, 761–777 (2023).
Yao, S. & Kendrick, K. M. How does oxytocin modulate human behavior?. Mol. Psychiatry https://doi.org/10.1038/s41380-025-02898-1 (2025).
Jeung-Maarse, H., Schmitgen, M. M., Schmitt, R., Bertsch, K. & Herpertz, S. C. Oxytocin effects on amygdala reactivity to angry faces in males and females with antisocial personality disorder. Neuropsychopharmacology 48, 946–953 (2023).
Whelan, T. P. et al. Editorial perspective: bridging the translational neuroscience gap in autism - development of the ‘shiftability’ paradigm. J. Child. Psychol. Psychiatry. 65, 862–865 (2024).
Bloomfield, M. A. P. et al. The effects of acute Cannabidiol on cerebral blood flow and its relationship to memory: an arterial spin labelling magnetic resonance imaging study. J. Psychopharmacol. 34, 981–989 (2020).
Bryant, J. E. et al. Ketamine induced changes in regional cerebral blood flow, interregional connectivity patterns, and glutamate metabolism. J. Psychiatr Res. 117, 108–115 (2019).
Martens, M. A. G. et al. Dopaminergic modulation of regional cerebral blood flow: an arterial spin labelling study of genetic and Pharmacological manipulation of COMT activity. Neuroimage 234, 117999 (2021).
Martins, D. et al. Effects of route of administration on oxytocin-induced changes in regional cerebral blood flow in humans. Nat. Commun. 11, 1–16 (2020).
Paloyelis, Y. et al. A Spatiotemporal profile of in vivo cerebral blood flow changes following intranasal Oxytocin in humans. Biol. Psychiatry. 79, 693–705 (2016).
Martins, D. et al. Less is more’: a dose-response account of intranasal Oxytocin pharmacodynamics in the human brain. Prog Neurobiol. 211, 1–17 (2022).
Tully, J. et al. A systematic review and meta-analysis of brain volume abnormalities in disruptive behaviour disorders, antisocial personality disorder and psychopathy. Nat. Mental Health. 1, 163–173 (2023).
De Brito, S. A., McDonald, D., Camilleri, J. A. & Rogers, J. C. Cortical and subcortical Gray matter volume in psychopathy: a voxel-wise meta-analysis. J. Abnorm. Psychol. 130, 627–640 (2021).
Coid, J. et al. The co-morbidity of personality disorder and clinical syndromes in prisoners. Criminal Behav. Mental Health. 19, 321–333 (2009).
Trull, T. J., Jahng, S., Tomko, R. L., Wood, P. K. & Sher, K. J. Revised NESARC personality disorder diagnoses: Gender, prevalence, and comorbidity with substance dependence disorders. J. Pers. Disord. 24, 412–426 (2010).
Deming, P., Heilicher, M. & Koenigs, M. How reliable are amygdala findings in psychopathy? A systematic review of MRI studies. Neurosci. Biobehav Rev. 142, 1–18 (2022).
Murphy, K. et al. Pulsed arterial spin labeling perfusion imaging at 3 T: estimating the number of subjects required in common designs of clinical trials. Magn. Reson. Imaging. 29, 1382–1389 (2011).
Cole, M. W., Bassett, D. S., Power, J. D., Braver, T. S. & Petersen, S. E. Intrinsic and task-evoked network architectures of the human brain. Neuron 83, 238–251 (2014).
Xue, G. et al. Common neural mechanisms underlying reversal learning by reward and punishment. PLoS One. 8, e82169 (2013).
Elster, E. M. et al. Altered neural responses to punishment learning in conduct disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. https://doi.org/10.1016/J.BPSC.2025.01.003 (2025).
Uddin, L. Q. Cognitive and behavioural flexibility: neural mechanisms and clinical considerations. Nat. Rev. Neurosci. 22, 167–179 (2021).
Hsu, M., Bhatt, M., Adolphs, R., Tranel, D. & Camerer, C. F. Neural systems responding to degrees of uncertainty in human decision-making. Sci. (1979). 310, 1680–1683 (2005).
Klein-Flügge, M. C., Bongioanni, A. & Rushworth, M. F. S. Medial and orbital frontal cortex in decision-making and flexible behavior. Neuron 110, 2743–2770 (2022).
Germuska, M. & Wise, R. G. Calibrated fMRI for mapping absolute CMRO2: practicalities and prospects. Neuroimage 187, 145–153 (2019).
Singh, N. et al. The effects of acute methylene blue administration on cerebral blood flow and metabolism in humans and rats. J. Cereb. Blood Flow Metab. 43, 95–105 (2023).
Sethi, A. et al. Emotional detachment in psychopathy: involvement of dorsal default-mode connections. Cortex 62, 11–19 (2015).
Deming, P. & Koenigs, M. Functional neural correlates of psychopathy: a meta-analysis of MRI data. Transl Psychiatry. 10, 1–8 (2020).
Dadario, N. B. & Sughrue, M. E. The functional role of the precuneus. Brain 146, 3598–3607 (2023).
Freeman, S. M. et al. The posteromedial region of the default mode network shows attenuated task-induced deactivation in psychopathic prisoners. Neuropsychology 29, 493–500 (2015).
Cavanna, A. E. & Trimble, M. R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129, 564–583 (2006).
Kable, J. W. & Glimcher, P. W. The neural correlates of subjective value during intertemporal choice. Nat. Neurosci. 10, 1625–1633 (2007).
Liu, X., Hairston, J., Schrier, M. & Fan, J. Common and distinct networks underlying reward Valence and processing stages: A meta-analysis of functional neuroimaging studies. Neurosci. Biobehav Rev. 35, 1219–1236 (2011).
Arioli, M., Cattaneo, Z., Ricciardi, E. & Canessa, N. Overlapping and specific neural correlates for empathizing, affective mentalizing, and cognitive mentalizing: A coordinate-based meta-analytic study. Hum. Brain Mapp. 42, 4777–4804 (2021).
Drayton, L. A., Santos, L. R. & Baskin-Sommers, A. R. Psychopaths fail to automatically take the perspective of others. Proc. Natl. Acad. Sci. U S A. 115, 3302–3307 (2018).
Deming, P. et al. Psychopathic traits linked to alterations in neural activity during personality judgments of self and others. Neuroimage Clin. 18, 575–581 (2018).
Deming, P. et al. Psychopathy is associated with fear-specific reductions in neural activity during affective perspective-taking. Neuroimage 223, 117342 (2020).
Newbury-Helps, J., Feigenbaum, J. & Fonagy, P. Offenders with antisocial personality disorder display more impairments in mentalizing. J. Pers. Disord. 31, 232–255 (2017).
Taubner, S., White, L. O., Zimmermann, J., Fonagy, P. & Nolte, T. Attachment-related mentalization moderates the relationship between psychopathic traits and proactive aggression in adolescence. J. Abnorm. Child. Psychol. 41, 929–938 (2013).
Bigot, A. et al. Confusing my viewpoint with his: altered self-other distinction performance in antisocial personality disorder. Personal Disord. 16, 110–121 (2025).
Quintana, D. S. et al. Oxytocin pathway gene networks in the human brain. Nat. Commun. 10, 1–12 (2019).
Balleine, B. W., Delgado, M. R. & Hikosaka, O. The role of the dorsal striatum in reward and Decision-Making. J. Neurosci. 27, 8161 (2007).
Malvaez, M. & Wassum, K. M. Regulation of habit formation in the dorsal striatum. Curr. Opin. Behav. Sci. 20, 67–74 (2018).
Haber, S. N. & Behrens, T. E. J. The neural network underlying Incentive-Based learning: implications for interpreting circuit disruptions in psychiatric disorders. Neuron 83, 1019–1039 (2014).
Kim, B. S. & Im, H. I. The role of the dorsal striatum in choice impulsivity. Ann. N Y Acad. Sci. 1451, 92–111 (2019).
Hawes, S. W. et al. Reward processing in children with disruptive behavior disorders and Callous-Unemotional traits in the ABCD study. Am. J. Psychiatry. 178, 333–342 (2021).
White, S. F. et al. Disrupted expected value and prediction error signaling in youths with disruptive behavior disorders during a passive avoidance task. Am. J. Psychiatry. 170, 315–323 (2013).
Finger, E. C. et al. Disrupted reinforcement signaling in the orbitofrontal cortex and caudate in youths with conduct disorder or oppositional defiant disorder and a high level of psychopathic traits. Am. J. Psychiatry. 168, 152–162 (2011).
Zhao, Z. et al. Oxytocin differentially modulates specific dorsal and ventral striatal functional connections with frontal and cerebellar regions. Neuroimage 184, 781–789 (2019).
Zhuang, Q. et al. Oxytocin-induced facilitation of learning in a probabilistic task is associated with reduced feedback- and error-related negativity potentials. J. Psychopharmacol. 35, 40–49 (2021).
Kruppa, J. A. et al. Neural modulation of social reinforcement learning by intranasal Oxytocin in male adults with high-functioning autism spectrum disorder: a randomized trial. Neuropsychopharmacology 44, 749–756 (2019).
Martins, D., Lockwood, P., Cutler, J., Moran, R. & Paloyelis, Y. Oxytocin modulates neurocomputational mechanisms underlying prosocial reinforcement learning. Prog Neurobiol. 213, 1–15 (2022).
Berends, Y. R. et al. Oxytocin and vasopressin in male forensic psychiatric patients with personality disorders and healthy controls. J. Forens Psychiatry Psychol. 33, 130–151 (2022).
Mitchell, I. J. et al. Psychopathic characteristics are related to high basal urinary Oxytocin levels in male forensic patients. J. Forensic Psychiatry Psychol. 24, 309–318 (2013).
Carson, D. S. et al. Cerebrospinal fluid and plasma Oxytocin concentrations are positively correlated and negatively predict anxiety in children. Mol. Psychiatry. 20, 1085–1090 (2014).
Martins, D., Gabay, A. S., Mehta, M. & Paloyelis, Y. Salivary and plasmatic Oxytocin are not reliable trait markers of the physiology of the Oxytocin system in humans. Elife 9, 1–19 (2020).
Seeley, S. H., Chou, Y. & O’Connor, M. F. Intranasal Oxytocin and OXTR genotype effects on resting state functional connectivity: A systematic review. Neurosci. Biobehav Rev. 95, 17–32 (2018).
Kou, J. et al. A randomized trial shows dose-frequency and genotype May determine the therapeutic efficacy of intranasal Oxytocin. Psychol. Med. 52, 1959–1968 (2022).
Mottolese, R., Redout́e, J., Costes, N., Le Bars, D. & Sirigu, A. Switching brain serotonin with Oxytocin. Proc. Natl. Acad. Sci. U S A. 111, 8637–8642 (2014).
Wang, J. et al. Effects of exogenous oxytocin on human brain function are regulated by oxytocin gene expression: a meta-analysis of 20 years of oxytocin neuroimaging and transcriptomic analyses. Neurosci. Biobehav Rev. https://doi.org/10.1016/J.NEUBIOREV.2025.106478 (2025).
Dumais, K. M., Kulkarni, P. P., Ferris, C. F. & Veenema, A. H. Sex differences in neural activation following different routes of Oxytocin administration in awake adult rats. Psychoneuroendocrinology 81, 52–62 (2017).
Stephens, J. A., Liu, P., Lu, H. & Suskauer, S. J. Cerebral blood flow after mild traumatic brain injury: associations between symptoms and Post-Injury perfusion. J. Neurotrauma. 35, 241 (2018).
Eklund, A., Nichols, T. E. & Knutsson, H. Cluster failure: why fMRI inferences for Spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. U S A. 113, 7900–7905 (2016).
Smith, S. M. & Nichols, T. E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44, 83–98 (2009).
Wechsler, D. Wechlser Abbreviated Scale of Intelligence, Second Edition (WASI-II) (NCS Pearson, 2011). https://doi.org/10.1177/0734282912467756
First, M. B., Williams, J. B. W., Karg, R. S. & Spitzer, R. L. User’s Guide To Structured Clinical Interview for DSM-5 Disorders, Clinical Version (American Psychiatric Association, 2016).
Cooke, D. J. & Michie, C. Psychopathy across cultures: North America and Scotland compared. J. Abnorm. Psychol. 108, 58–68 (1999).
MacDonald, E. et al. A review of safety, side-effects and subjective reactions to intranasal Oxytocin in human research. Psychoneuroendocrinology 36, 1114–1126 (2011).
Guastella, A. J. et al. Recommendations for the standardisation of Oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology 38, 612–625 (2013).
Alsop, D. C. et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn. Reson. Med. 73, 102–116 (2015).
McFarquhar, M. et al. Multivariate and repeated measures (MRM): A new toolbox for dependent and multimodal group-level neuroimaging data. Neuroimage 132, 373–389 (2016).
Martins, D. et al. Investigating resting brain perfusion abnormalities and disease target-engagement by intranasal Oxytocin in women with bulimia nervosa and binge-eating disorder and healthy controls. Transl Psychiatry. 10, 1–13 (2020).
Acknowledgements
Thank you to Dr Eleanor Hind for supporting with data collection. Thank you to the probation officers, especially David Bryan, for facilitating offender recruitment.
Funding
Funding for the research study was provided by Wellcome Clinical Research Training Fellowship grant for JT (grant no. 200099/S/15/S). Additional funding and financial support of the research team (JG, DMa, DMu, NG, NB) was provided by the National Institute for Health and Care Research (NIHR) Maudsley Biomedical Research Centre (BRC) and an Economic and Social Research Council (ESRC) grant to YP (grant no. ES/K009400/1). The views expressed are those of the author(s) and not necessarily those of the Wellcome Trust, ESRC, NIHR or the Department of Health and Social Care.
Author information
Authors and Affiliations
Contributions
Julia Griem: Conception, data acquisition, data analysis, data interpretation, manuscript drafting, manuscript revision, final approval of the version to be published; Daniel Martins: Data analysis, data interpretation, manuscript revision, final approval of the version to be published; John Tully: Conception, design, data acquisition, manuscript revision, final approval of the version to be published; Declan Murphy: Conception, design, manuscript revision, final approval of the version to be published; Yannis Paloyelis: Conception, design, data interpretation, manuscript revision, final approval of the version to be published; Nigel Blackwood: Conception, design, data interpretation, manuscript revision, final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Griem, J., Martins, D., Tully, J. et al. Resting-state brain function and its modulation by intranasal oxytocin in antisocial personality disorder with and without psychopathy. Sci Rep (2026). https://doi.org/10.1038/s41598-026-36661-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-36661-5