Abstract
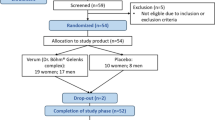
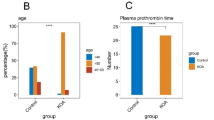
We investigated the associations of pro- and anti-inflammatory fatty acids (FAs) with cartilage degradation, functional limitations, pain, and psychological well-being in knee osteoarthritis (KOA). Fasting plasma samples were obtained from controls (n = 12) and from end-stage KOA patients at baseline (n = 13), and 3 months (n = 11) and 12 months (n = 9) after knee replacement surgery. FA composition in total lipids was analyzed with gas chromatography and mass spectrometry. Cartilage loss was determined by magnetic resonance imaging, and knee pain and disability by physical performance and quantitative sensory testing, neuromuscular examination, and several questionnaires. The associations between variables were tested with the univariate analysis of variance adjusted for age and body mass index. KOA was characterized with elevated baseline 16:1n-7 percentages, while the proportions of 24:0 decreased 12 months after surgery and those of 24:1n-9 decreased 3 and 12 months after surgery. Several FA variables, such as 20:3n-6, 20:4n-6, long-chain saturated FAs, and 24:1n-9, were associated with pain, stiffness, disability, pain self-efficacy, or mental health. Circulating FAs can predict KOA symptoms, independent of age and body adiposity, and provide promising targets to design novel pain treatments.
Similar content being viewed by others
Data availability
All relevant data analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- ANOVA:
-
Analysis of variance
- BAI:
-
Beck anxiety inventory
- BDI:
-
Beck depression inventory
- BMI:
-
Body mass index
- CE:
-
Cholesterol ester
- DA:
-
Discriminant analysis
- DI:
-
Desaturation index
- DMA:
-
Dimethyl acetal
- FA:
-
Fatty acid
- FAME:
-
Fatty acid methyl ester
- GLM:
-
Generalized linear model
- IL:
-
Interleukin
- KOA:
-
Knee osteoarthritis
- LICI:
-
Long-interval cortical inhibition
- LM:
-
Lipid mediator
- MRI:
-
Magnetic resonance imaging
- MUFA:
-
Monounsaturated fatty acid
- NSAID:
-
Non-steroidal anti-inflammatory drug
- nTMS:
-
Navigated transcranial magnetic stimulation
- OA:
-
Osteoarthritis
- PL:
-
Phospholipid
- PPT:
-
Pressure pain threshold
- PSEQ:
-
Pain self-efficacy questionnaire
- PUFA:
-
Polyunsaturated fatty acid
- RA:
-
Rheumatoid arthritis
- rMT:
-
Resting motor threshold
- ROM:
-
Range of motion
- rs :
-
Spearman correlation coefficient
- Rv:
-
Resolvin
- SE:
-
Standard error of the mean
- SF:
-
Synovial fluid
- SFA:
-
Saturated fatty acid
- TKA:
-
Total knee arthroplasty
- TNF-α :
-
Tumor necrosis factor-α
- TPD:
-
Two-point discrimination
- VAS:
-
Visual analog scale
- WOMAC:
-
Western Ontario and McMaster Universities Osteoarthritis Index
References
Fayet, M. & Hagen, M. Pain characteristics and biomarkers in treatment approaches for osteoarthritis pain. Pain Manag. 11, 59–73 (2021).
Fu, K., Robbins, S. R. & McDougall, J. J. Osteoarthritis: The genesis of pain. Rheumatology 57(Suppl. 4), iv43–iv50 (2018).
Bedson, J. & Croft, P. R. The discordance between clinical and radiographic knee osteoarthritis: A systematic search and summary of the literature. BMC Musculoskelet. Disord. 9, 116 (2008).
Thudium, C. S., Löfvall, H., Karsdal, M. A., Bay-Jensen, A.-C. & Bihlet, A. R. Protein biomarkers associated with pain mechanisms in osteoarthritis. J. Proteomics 190, 55–66 (2019).
Mustonen, A.-M. & Nieminen, P. Fatty acids and oxylipins in osteoarthritis and rheumatoid arthritis: A complex field with significant potential for future treatments. Curr. Rheumatol. Rep. 23, 41 (2021).
Navarini, L., Afeltra, A., Gallo Afflitto, G. & Margiotta, D. P. E. Polyunsaturated fatty acids: Any role in rheumatoid arthritis?. Lipids Health Dis. 16, 197 (2017).
Bahamondes, M. A., Valdés, C. & Moncada, G. Effect of omega-3 on painful symptoms of patients with osteoarthritis of the synovial joints: Systematic review and meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 132, 297–306 (2021).
Sibille, K. T. et al. Omega-6:omega-3 PUFA ratio, pain, functioning, and distress in adults with knee pain. Clin. J. Pain 34, 182–189 (2018).
Lourdudoss, C. et al. Dietary intake of polyunsaturated fatty acids and pain in spite of inflammatory control among methotrexate-treated early rheumatoid arthritis patients. Arthritis Care Res. 70, 205–212 (2018).
Loef, M. et al. The association of the lipid profile with knee and hand osteoarthritis severity: The IMI-APPROACH cohort. Osteoarthritis Cartilage 30, 1062–1069 (2022).
Loef, M. et al. The association of plasma fatty acids with hand and knee osteoarthritis: The NEO study. Osteoarthritis Cartilage 28, 223–230 (2020).
Felson, D. T. et al. Fatty acids and osteoarthritis: The MOST study. Osteoarthritis Cartilage 29, 973–978 (2021).
Sekar, S. et al. Dietary saturated fatty acids modulate pain behaviour in trauma-induced osteoarthritis in rats. Nutrients 12, 509 (2020).
Pousinis, P. et al. Lipidomic identification of plasma lipids associated with pain behaviour and pathology in a mouse model of osteoarthritis. Metabolomics 16, 32 (2020).
Barden, A. E. et al. Specialised pro-resolving mediators of inflammation in inflammatory arthritis. Prostaglandins Leukot. Essent. Fatty Acids 107, 24–29 (2016).
Xu, Z.-Z. et al. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat. Med. 16, 592–597 (2010).
Lima-Garcia, J. F. et al. The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br. J. Pharmacol. 164, 278–293 (2011).
Mustonen, A.-M. et al. Distinct fatty acid signatures in infrapatellar fat pad and synovial fluid of patients with osteoarthritis versus rheumatoid arthritis. Arthritis Res. Ther. 21, 124 (2019).
Mustonen, A.-M. et al. Tetraspanin profiles of serum extracellular vesicles reflect functional limitations and pain perception in knee osteoarthritis. Arthritis Res. Ther. 26, 33 (2024).
Bellamy, N., Buchanan, W. W., Goldsmith, C. H., Campbell, J. & Stitt, L. W. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. Rheumatol. 15, 1833–1840 (1988).
Soininen, J. V., Paavolainen, P. O., Gronblad, M. A. & Kaapa, E. H. Validation study of a Finnish version of the Western Ontario and McMasters University osteoarthritis index. Hip Int. 18, 108–111 (2008).
Hawker, G. A., Mian, S., Kendzerska, T. & French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 63(Suppl. 11), S240–S252 (2011).
Freynhagen, R., Baron, R., Gockel, U. & Tölle, T. R. painDETECT: A new screening questionnaire to identify neuropathic components in patients with back pain. Curr. Med. Res. Opin. 22, 1911–1920 (2006).
Nicholas, M. K. The pain self-efficacy questionnaire: Taking pain into account. Eur. J. Pain 11, 153–163 (2007).
Hays, R. D. & Morales, L. S. The RAND-36 measure of health-related quality of life. Ann. Med. 33, 350–357 (2001).
Beck, A. T., Steer, R. A., Ball, R., Ciervo, C. A. & Kabat, M. Use of the Beck Anxiety and Depression Inventories for primary care with medical outpatients. Assessment 4, 211–219 (1997).
Liikavainio, T., Lyytinen, T., Tyrväinen, E., Sipilä, S. & Arokoski, J. P. Physical function and properties of quadriceps femoris muscle in men with knee osteoarthritis. Arch. Phys. Med. Rehabil. 89, 2185–2194 (2008).
Dobson, F. et al. OARSI recommended performance-based tests to assess physical function in people diagnosed with hip or knee osteoarthritis. Osteoarthritis Cartilage 21, 1042–1052 (2013).
Suokas, A. K. et al. Quantitative sensory testing in painful osteoarthritis: A systematic review and meta-analysis. Osteoarthritis Cartilage 20, 1075–1085 (2012).
Wylde, V., Palmer, S., Learmonth, I. D. & Dieppe, P. Somatosensory abnormalities in knee OA. Rheumatology 51, 535–543 (2012).
Stanton, T. R. et al. Tactile acuity is disrupted in osteoarthritis but is unrelated to disruptions in motor imagery performance. Rheumatology 52, 1509–1519 (2013).
The R Foundation. The R project for statistical computing. Vienna, Austria. https://www.r-project.org (2023).
Wei, T. & Simko, V. R package ‘corrplot’: Visualization of a correlation matrix, version 0.92. https://github.com/taiyun/corrplot (2021).
Kim, S. et al. Metabolite profiles of synovial fluid change with the radiographic severity of knee osteoarthritis. Joint Bone Spine 84, 605–610 (2017).
Plumb, M. S. & Aspden, R. M. High levels of fat and (n-6) fatty acids in cancellous bone in osteoarthritis. Lipids Health Dis. 3, 12 (2004).
Van de Vyver, A. et al. Synovial fluid fatty acid profiles differ between osteoarthritis and healthy patients. Cartilage 11, 473–478 (2020).
Song, J., Kang, Y.-H., Yoon, S., Chun, C.-H. & Jin, E.-J. HIF-1α:CRAT:miR-144-3p axis dysregulation promotes osteoarthritis chondrocyte apoptosis and VLCFA accumulation. Oncotarget 8, 69351–69361 (2017).
Wu, C.-L., Kimmerling, K. A., Little, D. & Guilak, F. Serum and synovial fluid lipidomic profiles predict obesity-associated osteoarthritis, synovitis, and wound repair. Sci. Rep. 7, 44315 (2017).
Sanders, A. E. et al. Circulating polyunsaturated fatty acids, pressure pain thresholds, and nociplastic pain conditions. Prostaglandins Leukot. Essent. Fatty Acids 184, 102476 (2022).
Ramsden, C. et al. Do omega-6 and trans fatty acids play a role in complex regional pain syndrome?. A pilot study. Pain Med. 11, 1115–1125 (2010).
Frommer, K. W. et al. Free fatty acids: Potential proinflammatory mediators in rheumatic diseases. Ann. Rheum. Dis. 74, 303–310 (2015).
Khoury, S. et al. Identification of lipid biomarkers for chronic joint pain associated with different joint diseases. Biomolecules 13, 342 (2023).
Poitelon, Y., Kopec, A. M. & Belin, S. Myelin fat facts: An overview of lipids and fatty acid metabolism. Cells 9, 812 (2020).
Li, Q., Chen, J., Yu, X. & Gao, J.-M. A mini review of nervonic acid: Source, production, and biological functions. Food Chem 301, 125286 (2019).
Phung, N. V. et al. Nervonic acid and its sphingolipids: Biological functions and potential food applications. Crit. Rev. Food Sci. Nutr. 64, 8766–8785 (2024).
Sastry, P. S. Lipids of nervous tissue: Composition and metabolism. Prog. Lipid Res. 24, 69–176 (1985).
Hill, C. L. et al. Fish oil in knee osteoarthritis: A randomised clinical trial of low dose versus high dose. Ann. Rheum. Dis. 75, 23–29 (2016).
Keen, H. et al. Treatment of diabetic neuropathy with γ-linolenic acid. Diabetes Care 16, 8–15 (1993).
Clough, G. F. et al. Higher body fat percentage is associated with enhanced temperature perception in NAFLD: Results from the randomised Wessex Evaluation of fatty Liver and Cardiovascular markers in NAFLD with OMacor thErapy trial (WELCOME) trial. Diabetologia 59, 1422–1429 (2016).
Kittelson, A. J., Thomas, A. C., Kluger, B. M. & Stevens-Lapsley, J. E. Corticospinal and intracortical excitability of the quadriceps in patients with knee osteoarthritis. Exp. Brain Res. 232, 3991–3999 (2014).
Bazinet, R. P. & Layé, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 15, 771–785 (2014).
Di Miceli, M., Bosch-Bouju, C. & Layé, S. PUFA and their derivatives in neurotransmission and synapses: A new hallmark of synaptopathies. Proc. Nutr. Soc. 79, 388–403 (2020).
Chen, R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp. Brain Res. 154, 1–10 (2004).
Burston, J. J. et al. The impact of anxiety on chronic musculoskeletal pain and the role of astrocyte activation. Pain 160, 658–669 (2019).
Yary, T. et al. Serum dihomo-γ-linolenic acid level is inversely associated with the risk of depression. A 21-year follow-up study in general population men. J. Affect. Disord. 213, 151–155 (2017).
Kelaiditis, C. F., Gibson, E. L. & Dyall, S. C. Effects of long-chain omega-3 polyunsaturated fatty acids on reducing anxiety and/or depression in adults; A systematic review and meta-analysis of randomised controlled trials. Prostaglandins Leukot. Essent. Fatty Acids 192, 102572 (2023).
Wang, S.-T. & Ni, G.-X. Depression in osteoarthritis: Current understanding. Neuropsychiatr. Dis. Treat. 18, 375–389 (2022).
Prete, P. E., Gurakar-Osborne, A. & Kashyap, M. L. Synovial fluid lipids and apolipoproteins: A contemporary perspective. Biorheology 32, 1–16 (1995).
Laurent, T. C. & Fraser, J. R. E. Hyaluronan. FASEB J. 6, 2397–2404 (1992).
Deng, W. et al. Effect of omega-3 polyunsaturated fatty acids supplementation for patients with osteoarthritis: A meta-analysis. J. Orthop. Surg. Res. 18, 381 (2023).
Sun, Q., Ma, J., Campos, H., Hankinson, S. E. & Hu, F. B. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am. J. Clin. Nutr. 86, 74–81 (2007).
Acknowledgements
Taija Hukkanen is greatly acknowledged for the blood sampling and transmethylation of the samples, and Meri Julkunen and Anna-Leena Voutilainen for coordinating the study. We are also thankful for Heikki Kyykallio, Johanna Matilainen, and Tommi Paakkonen for technical assistance.
Funding
Financial support for the study was provided by the Research Council of Finland (grant #322429). The funding source had no involvement in the study design, in the collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
PN, PJ, HK, and JA designed and coordinated the study. HK collected the samples, and LS, JR, and PJ conducted the physical analyses and LK the physiatric measurements. JR performed the magnetic resonance imaging, and AE determined the thickness of articular cartilage. RK and SPS performed the fatty acid analyses. PN integrated the chromatograms, and PN, A-MM, and JK conducted the statistical analyses and prepared the images. A-MM drafted the manuscript. All authors revised the draft critically and read and approved the final submitted manuscript.
Corresponding author
Ethics declarations
Competing interests
Petro Julkunen reports unrelated consulting fees and a patent with Nexstim Plc (Helsinki, Finland), a manufacturer of navigated transcranial magnetic stimulation systems. Jusa Reijonen reports unrelated consulting fees with Nexstim Plc. The other authors have no relevant financial or non-financial interests to disclose.
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Ethical approval was obtained from the Ethical Committee of Kuopio University Hospital (decision #140/2017, amendment 8/2020). Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mustonen, AM., Säisänen, L., Karttunen, L. et al. Plasma fatty acids reflect pain, disability, and psychological well-being in knee osteoarthritis in a longitudinal study with joint replacement surgery. Sci Rep (2026). https://doi.org/10.1038/s41598-026-36812-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-36812-8