Abstract
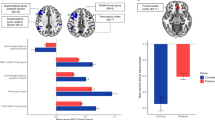
To tackle the disease-related process in early pre-dementia Lewy Body Dementia, we investigated the changes of functional brain networks and their cognitive relevance. A cohort of 38 Mild Cognitive Impairment with Lewy Bodies (MCI-LB) subjects and one of 24 healthy controls (HC) underwent neuropsychological assessment and resting state (RS) functional and structural MRI. Functional connectivity (FC) between ROIs belonging to a set of RS networks, including the Salience Network (SN), Fronto-Parietal (FPN), Default Mode (DMN), Dorsal and Ventral Attention (DAN and VAN), Somato-Motor (SMN), Visual (VN) and Language (LN) was estimated and compared between cohorts. Finally, neuropsychological scores were correlated with FC of MCI-LB and HC separately. Compared to HC, MCI-LB exhibited lower FC between DAN, FPN and LN. Higher inter-network FC was found between FPN and SN, FPN and DMN, SN and SMN and DAN and SMN. In MCI-LB the correlational analysis revealed significant positive and negative associations between cognitive performance and FC values between nodes. In conclusion, we found a possible compensation mechanism between nodes in SN and FPN, and FPN and DMN following disconnection between the control system of the FPN and the top down attention system. The complex compensatory mechanisms involving multiple networks may not be efficient to counteract the cognitive impairment in MCI-LB. Overall, in MCI-LB we found an aberrant engagement of the networks that are not primarily involved in the performance of specific tasks.
Similar content being viewed by others
Data availability
All relevant raw data will be available upon request to any researcher wishing to use them for non-commercial purposes, for privacy reasons.For access to the raw data, please contact Prof. Irena Rektorova at irena.rektorova@fnusa.cz.
References
Donaghy, P. C. et al. Research diagnostic criteria for mild cognitive impairment with lewy bodies: A systematic review and meta-analysis. Alzheimers Dement. 19 (7), 3186–3202 (2023).
McKeith, I. G. et al. Research criteria for the diagnosis of prodromal dementia with lewy bodies. Neurol. 28 Avr. 94 (17), 743–755 (2020).
Iranzo, A. et al. Detection of α-synuclein in CSF by RT-QuIC in patients with isolated rapid-eye-movement sleep behaviour disorder: a longitudinal observational study. Lancet Neurol. Mars. 20 (3), 203–212 (2021).
Yau, Y. et al. Network connectivity determines cortical thinning in early parkinson’s disease progression. Nat. Commun. 2 Janv. 9 (1), 12 (2018).
Rahayel, S. et al. Differentially targeted seeding reveals unique pathological alpha-synuclein propagation patterns. Brain J. Neurol. 3 Juin. 145 (5), 1743–1756 (2022).
McKeith, I. G. et al. Diagnosis and management of dementia with lewy bodies: fourth consensus report of the DLB consortium. Neurol. 4 Juill. 89 (1), 88–100 (2017).
Ferman, T. J. et al. Nonamnestic mild cognitive impairment progresses to dementia with lewy bodies. Neurol. 3 déc. 81 (23), 2032–2038 (2013).
Ferman, T. J. et al. Neuropsychological differentiation of dementia with lewy bodies from normal aging and alzheimer’s disease. Clin. Neuropsychol. déc. 20 (4), 623–636 (2006).
Ciafone, J., Little, B., Thomas, A. J. & Gallagher, P. The neuropsychological profile of mild cognitive impairment in lewy body dementias. J. Int. Neuropsychol. Soc. févr. 26 (2), 210–225 (2020).
Lowther, E. R., O’Brien, J. T., Firbank, M. J. & Blamire, A. M. Lewy body compared with alzheimer dementia is associated with decreased functional connectivity in resting state networks. Psychiatry Res. 30 Sept. 223 (3), 192–201 (2014).
Schumacher, J. et al. Functional connectivity in dementia with lewy bodies: A within- and between-network analysis. Hum. Brain Mapp. Mars. 39 (3), 1118–1129 (2018).
Schumacher, J. et al. Functional connectivity in mild cognitive impairment with lewy bodies. J. Neurol. déc. 268 (12), 4707–4720 (2021).
Peraza, L. R. et al. fMRI resting state networks and their association with cognitive fluctuations in dementia with lewy bodies. NeuroImage Clin. 28 Mars. 4, 558–565 (2014).
Kenny, E. R., Blamire, A. M., Firbank, M. J. & O’Brien, J. T. Functional connectivity in cortical regions in dementia with lewy bodies and alzheimer’s disease. Brain févr. 135 (2), 569–581 (2012).
Franciotti, R. et al. Default network is not hypoactive in dementia with fluctuating cognition: an alzheimer disease/dementia with lewy bodies comparison. Neurobiol. Aging Avr. 34 (4), 1148–1158 (2013).
Menon, V. & Uddin, L. Q. Saliency, switching, attention and control: a network model of Insula function. Brain Struct. Funct. Juin. 214 (5–6), 655–667 (2010).
Crottaz-Herbette, S. & Menon, V. Where and when the anterior cingulate cortex modulates attentional response: combined fMRI and ERP evidence. J. Cogn. Neurosci. Mai. 18 (5), 766–780 (2006).
Kobeleva, X. et al. Divergent functional connectivity during attentional processing in lewy body dementia and alzheimer’s disease. Cortex J. Devoted Study Nerv. Syst. Behav. Juill. 92, 8–18 (2017).
Corbetta, M. & Shulman, G. L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. Mars. 3 (3), 201–215 (2002).
Alves, P. N., Forkel, S. J., Corbetta, M. & Thiebaut de Schotten, M. The subcortical and neurochemical organization of the ventral and dorsal attention networks. Commun. Biol. 7 déc. 5 (1), 1–14 (2022).
Habich, A., Wahlund, L. O., Westman, E., Dierks, T. & Ferreira, D. Dis-)Connected Dots in dementia with lewy Bodies—A systematic review of connectivity studies. Mov. Disord. 38 (1), 4–15 (2023).
Mehraram, R. et al. Functional and structural brain network correlates of visual hallucinations in lewy body dementia. Brain 9 Mars. 145 (6), 2190–2205 (2022).
Han, S. W., Eaton, H. P. & Marois, R. Functional fractionation of the Cingulo-opercular network: alerting Insula and updating cingulate. Cereb. Cortex 1 Juin. 29 (6), 2624–2638 (2019).
Roquet, D. et al. Insular atrophy at the prodromal stage of dementia with lewy bodies: a VBM DARTEL study. Sci. Rep. 25 août. 7, 9437 (2017).
Goulden, N. et al. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. NeuroImage 1 oct. 99, 180–190 (2014).
Shine, J. M. et al. The role of dysfunctional attentional control networks in visual misperceptions in parkinson’s disease. Hum. Brain Mapp. Mai. 35 (5), 2206–2219 (2014).
Litvan, I. et al. Diagnostic criteria for mild cognitive impairment in parkinson’s disease: movement disorder society task force guidelines. Mov. Disord Off J. Mov. Disord Soc. Mars. 27 (3), 349–356 (2012).
Železníková, Ž. et al. Early changes in the locus coeruleus in mild cognitive impairment with lewy bodies. Mov. Disord Off J. Mov. Disord Soc. févr. 40 (2), 276–284 (2025).
Nasreddine, Z. S. et al. The Montreal cognitive Assessment, moca: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. Avr. 53 (4), 695–699 (2005).
Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The unified parkinson’s disease rating scale (UPDRS): status and recommendations. Mov. Disord Off J. Mov. Disord Soc. Juill. 18 (7), 738–750 (2003).
Ferman, T. J. et al. DLB fluctuations: specific features that reliably differentiate DLB from AD and normal aging. Neurol. 27 Janv. 62 (2), 181–187 (2004).
Yesavage, J. A. et al. Development and validation of a geriatric depression screening scale: a preliminary report. J. Psychiatr Res. 1983. 17 (1), 37–49 (1982).
Stiasny-Kolster, K. et al. The REM sleep behavior disorder screening questionnaire–a new diagnostic instrument. Mov. Disord Off J. Mov. Disord Soc. déc. 22 (16), 2386–2393 (2007).
Cummings, J. L. et al. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurol. déc. 44 (12), 2308–2314 (1994).
Johns, M. W. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. déc. 14 (6), 540–545 (1991).
Benedict, R. H. B. Brief Visuospatial Memory Test Revised Professional Manual. Odessa, FL Psychological Assessment Resources. - References - Scientific Research Publishing [Internet]. [cité 7 déc 2025]. Disponible sur: (1997). https://www.scirp.org/reference/referencespapers?referenceid=1738365
Bezdicek, O. et al. Development, validity, and normative data study for the 12-word Philadelphia verbal learning test [czP(r)VLT-12] among older and very old Czech adults. Clin. Neuropsychol. 28 (7), 1162–1181 (2014).
Taub, G. E., McGrew, K. S. & Witta, E. L. A confirmatory analysis of the factor structure and cross-age invariance of the Wechsler adult intelligence Scale-Third edition. Psychol. Assess. Mars. 16 (1), 85–89 (2004).
Nikolai, T. et al. Tests of verbal Fluency, Czech normative study in older patients. Čes Slov. Neurol. Neurochir. 29 Mai. 78/111, 292–299 (2015).
Moeller, S. et al. Multiband Multislice GE-EPI at 7 Tesla, With 16-Fold Acceleration Using Partial Parallel Imaging With Application to High Spatial and Temporal Whole-Brain FMRI. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. mai. 63 (5):1144–53. (2010).
Feinberg, D. A. et al. Multiplexed echo planar imaging for Sub-Second whole brain FMRI and fast diffusion imaging. PLOS ONE Dic. 5 (12), e15710 (2010).
Whitfield-Gabrieli, S. & Nieto-Castanon, A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2 (3), 125–141 (2012).
Friston, K. J. et al. Analysis of fMRI time-series revisited. NeuroImage Mars. 2 (1), 45–53 (1995).
Spadone, S. et al. Dynamic brain States in Spatial neglect after stroke. Front Syst Neurosci [Internet]. 2 mai 2023 [cité 7 déc 2025];17. Disponible sur: https://www.frontiersin.org/journals/systems-neuroscience/articles/https://doi.org/10.3389/fnsys.2023.1163147/full
Baldassarre, A., Filardi, M. S., Spadone, S., Penna, S. D. & Committeri, G. Distinct connectivity profiles predict different in-time processes of motor skill learning. NeuroImage 1 Sept. 238, 118239 (2021).
Marek, S. & Dosenbach, N. U. F. The frontoparietal network: function, electrophysiology, and importance of individual precision mapping. Dialogues Clin. Neurosci. Juin. 20 (2), 133–140 (2018).
Spadone, S. et al. Dynamic brain States in Spatial neglect after stroke. Front. Syst. Neurosci. [Internet]. https://doi.org/10.3389/fnsys.2023.1163147 (2023). [cité 18 juill 2023];17. Disponible sur: https://www.frontiersin.org/articles/
Xia, M., Wang, J. & He, Y. BrainNet viewer: A network visualization tool for human brain connectomics. PLOS ONE 4 Juill. 8 (7), e68910 (2013).
Ahrens, M. M., Veniero, D., Freund, I. M., Harvey, M. & Thut, G. Both dorsal and ventral attention network nodes are implicated in exogenously driven visuospatial anticipation. Cortex J. Devoted Study Nerv. Syst. Behav. août. 117, 168–181 (2019).
Greicius, M. D., Krasnow, B., Reiss, A. L. & Menon, V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci. 7 janv. ;100(1):253–8. (2003).
Chabran, E. et al. Changes in gray matter volume and functional connectivity in dementia with Lewy bodies compared to Alzheimer’s disease and normal aging: implications for fluctuations. Alzheimers Res Ther. 6 janv. ;12(1):9. (2020).
Sadaghiani, S. & D’Esposito, M. sept. Functional characterization of the Cingulo-Opercular network in the maintenance of tonic Alertness. Cereb cortex. 25(9):2763–2773. (2015).
Katsuki, F. & Constantinidis, C. Bottom-up and top-down attention: different processes and overlapping neural systems. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry oct. 20 (5), 509–521 (2014).
Chen, W., Liang, J., Wu, Q. & Han, Y. Anterior cingulate cortex provides the neural substrates for feedback-driven iteration of decision and value representation. Nat. Commun. 17 Juill. 15 (1), 6020 (2024).
Habich, A. et al. Grey matter networks in women and men with dementia with lewy bodies. Npj Park Dis. 13 Avr. 10 (1), 84 (2024).
Tang, S. et al. Large-scale network dysfunction in α-Synucleinopathy: A meta-analysis of resting-state functional connectivity. eBioMedicine [Internet]. 1 mars 2022 [cité 23 sept 2025];77. Disponible sur: https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(22)00099-8/fulltext
Kucikova, L. et al. A systematic literature review of fMRI and EEG resting-state functional connectivity in dementia with lewy bodies: underlying mechanisms, clinical manifestation, and methodological considerations. Ageing Res. Rev. Janv. 93, 102159 (2024).
Liu, L. et al. oct. The two-brain approach reveals the active role of task-deactivated default mode network in speech comprehension. Cereb cortex. 32(21):4869–4884. (2022).
Ren, Z. et al. The Different Brain Mechanisms of Object and Spatial Working Memory: Voxel-Based Morphometry and Resting-State Functional Connectivity. Front Hum Neurosci [Internet]. 19 juill 2019 [cité 1 mars 2025];13. Disponible sur: https://www.frontiersin.org/journals/human-neuroscience/articles/https://doi.org/10.3389/fnhum.2019.00248/full
Delli Pizzi, S. et al. Relevance of subcortical visual pathways disruption to visual symptoms in dementia with lewy bodies. Cortex 1 oct. 59, 12–21 (2014).
Reineberg, A. E., Andrews-Hanna, J. R., Depue, B. E., Friedman, N. P. & Banich, M. T. Resting-state networks predict individual differences in common and specific aspects of executive function. NeuroImage 1 Janv. 104, 69–78 (2015).
Reineberg, A. E., Gustavson, D. E., Benca, C., Banich, M. T. & Friedman, N. P. The relationship between resting state network connectivity and individual differences in executive functions. Front. Psychol. 5 Sept. 9, 1600 (2018).
Zhao, J. et al. Age-Related Decreases in Interhemispheric Resting-State Functional Connectivity and Their Relationship With Executive Function. Front Aging Neurosci [Internet]. 26 févr 2020 [cité 10 juin 2024];12. Disponible sur: https://www.frontiersin.org/articles/https://doi.org/10.3389/fnagi.2020.00020
Ye, Z. & Zhou, X. Executive control in Language processing. Neurosci. Biobehav Rev. 1 Sept. 33 (8), 1168–1177 (2009).
Hyde, J. S. & Linn, M. C. Gender differences in verbal ability: A meta-analysis. Psychol. Bull. 104 (1), 53–69 (1988).
Yeager, B. E., Twedt, H. P., Bruss, J., Schultz, J. & Narayanan, N. S. Cortical and subcortical functional connectivity and cognitive impairment in parkinson’s disease. NeuroImage Clin. 42, 103610 (2024).
Majerus, S. et al. Attention supports verbal short-term memory via competition between dorsal and ventral attention networks. Cereb Cortex N Y N 1991. mai. ;22(5):1086–97. (2012).
Jones, S. D. & Westermann, G. Under-Resourced or overloaded? Rethinking working memory deficits in developmental Language disorder. Psychol. Rev. Nov. 129 (6), 1358–1372 (2022).
Majerus, S. et al. The commonality of neural networks for verbal and visual short-term memory. J. Cogn. Neurosci. Nov. 22 (11), 2570–2593 (2010).
Clark, V. P., Fannon, S., Lai, S., Benson, R. & Bauer, L. Responses to rare visual target and distractor stimuli using Event-Related fMRI. J. Neurophysiol. Mai. 83 (5), 3133–3139 (2000).
Bartolomeo, P., Thiebaut De Schotten, M. & Chica, A. B. Brain networks of visuospatial attention and their disruption in visual neglect. Front Hum Neurosci [Internet]. 4 mai 2012 [cité 23 sept 2025];6. Disponible sur: https://www.frontiersin.org/journals/human-neuroscience/articles/https://doi.org/10.3389/fnhum.2012.00110/full
Birn, R. M. et al. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. NeuroImage déc. 83, 550–558 (2013).
Funding
The work was supported by the Ministry of Health of the Czech Republic (grant NU21J-04-00077) and by an EU Joint Program-Neurodegenerative Disease (JPND) project entitled: TACKLing the challenges of PREsymptomatic sporadic Dementia (TACKL-PRED), project no. 8F22005. We acknowledge the core facility MAFIL, supported by the Czech-BioImaging large RI project (LM2023050 funded by MEYS CR), part of the Euro-BioImaging (www.eurobioimaging.eu) ALM and Medical Imaging Node (Brno, CZ), for their support with obtaining scientific data presented in this paper. Supported by project no. LX22NPO5107 (MEYS): Funded by the European Union – Next Generation EU.
Author information
Authors and Affiliations
Contributions
Valeria Onofrj (1ABC, 2 C, 3 A), Raffaella Franciotti (1AC, 2AC, 3B), Kristina Mitterova (1 C, 2 C, 3B), Lubos Brabenec (1 C, 2 C, 3B), Martin Gajdos (1B, 2 C, 3B), Ivona Moravkova (1 C, 2 C, 3B), Antonio Ferretti (1 C, 2 C, 3B), Sara Spadone (1 C, 2AB, 3B), Caterina Padulo (1 C, 2 C), Antonello Baldassarre (1 C, 2AB, 3B), Stefano Sensi (1 C, 2 C, 3B), Irena Rektorova (1AB, 2AC, 3B).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Authors roles
1) Research project: (A) Conception, (B) Organization, (C) Execution; 2) Statistical analysis: A.
Design, B. Execution, C. Review and Critique; 3) Manuscript: A. Writing of the first draft, B.
Review and Critique.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Onofrj, V., Franciotti, R., Mitterova, K. et al. MCI-LB brain networks reorganization in relation to specific cognitive domains deficits. Sci Rep (2026). https://doi.org/10.1038/s41598-026-36953-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-36953-w